
������ָ��ս�ˮ������(pHС��5.6)������ط������pH������2.1(ʳ��pH��3)���γ��������Ҫ�Ǵ����е�SO2�͵���������ǵ���Ҫ��Դ��ú��ʯ�͵�ȼ�գ�ȫ����ÿ����1.5�ڶ�SO2���ŷ�����
(1)SO2���ڿ������ܹ��ձ���������������ˮ�γ����ꡣ��д����������ѧ��Ӧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
(2)�����ŷŵ�β�������᳧�ͻ��ʳ��ķ��������������ȫ����ÿ���ŷ���Լ5��107 kg��NO2����ˮ����________�ᡣ
(3)����ɵ��µ�Σ����(����)
A����ʴ������ B��������ľ��ή
C����ɺ����ֺ� D���������
(4)Ϊ�˼���������γɣ��������SO2���ŷ�������ȼ���е��������________���Է����еĵ����������________��
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������HCl�� ��NaOH ����CH3COOH ����NH3��H2O�� ��CH3COONa ����NH4Cl
��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01mol/L HCl��Һ��PH= ��PH=11��CH3COONa��Һ����ˮ���������c(OH��) = ��
��3������PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
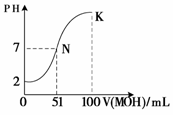 ��4�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
��4�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
��N���Ӧ����Һ�У�c(M��) c(A��)������ڡ����ڡ�С�ڡ�����
��K���Ӧ����Һ�У�c(M��)��c(MOH)= mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����Ҫ�Ļ���ԭ�ϡ���ҵ�ϳ��÷�Ӧ3Cl2��8NH3===N2��6NH4Cl���������ܵ��Ƿ�©�������ݲ����еķ�Ӧ�ش��������⣺
(1)��������________����ԭ����________��
(2)�������뱻�����Ļ�ԭ�����Ӹ�����Ϊ________��
(3)����68 g NH3�μӷ�Ӧʱ��������������Ϊ______g�����ɵĻ�ԭ����Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
K2Cr2O7��H2O2��H2SO4��Һ�л��ʱ���ɹ۲쵽������������
�ٻ�Ϻ�5��10���ڣ���Һ�ɳ�ɫ��Ϊ����ɫ��
����80��150�����ɰ���ɫ��Ϊ��ɫ�����ͬʱ�ų����ݡ���֮��Ӧ��������ӦΪ��
A��Cr2O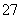 ��H2O2��H���D��CrO5��H2O
��H2O2��H���D��CrO5��H2O
B��CrO5��H���D��Cr3����O2����H2O
(1)��֪��ӦA����������ԭ��Ӧ����CrO5��CrԪ�صļ�̬Ϊ____________��
(2)��ӦB�Ƿ�����������ԭ��Ӧ��
��____________����������____________________________________________��
(3)����ӦA�ͷ�ӦB��ӣ����Եõ�һ���ܻ�ѧ����ʽ�����ܷ�Ӧ����������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϊ�������������;���ɲ��õĴ�ʩ��(����)
������ú��ȼ�ϡ��ڰѹ�����±��ߡ���ȼ�����������ữ�������м�ʯ�ҡ��ݿ�������Դ
A���٢ڢ� B���ڢۢܢ�
C���٢ۢ� D���٢ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ���䷴Ӧ���������ɫ���졢��֡����ơ���ɫ˳�����е���(����)
�ٽ������ڴ�����ȼ�ա���FeSO4��Һ�е���NaOH��Һ�����ڿ����з���һ��ʱ�䡡��FeCl3��Һ�е���KSCN��Һ������ˮ����ͭ����ҽ�þƾ���
A���ڢۢ٢� B���ۢڢ٢�
C���ۢ٢ڢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ����ʺ���;�������(����)
A����������һ�ֺ���ɫ��ĩ���������������Ϳ��
B����������һ���ͻ���ϣ������������ͻ��������ͻ�ש
C������ͭ�ʺ�ɫ������Ϊ�����մɵĺ�ɫ����
D������������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���½�������ʵ�飺�ס��ҡ�����ȡ30 mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһþ���Ͻ��ĩ���й������б����£�
| ʵ����� | �� | �� | �� |
| �Ͻ�����/mg | 510 | 765 | 918 |
| �������/mL | 560 | 672 | 672 |
(1)��������ʵ���Ũ���Ƕ��٣�
(2)��Ͻ���þ����������������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤��������ֵ������˵����ȷ����
A��28 g��C2H4��C3H6��ɵĻ�����к�����ԭ�ӵ���ĿΪ4 NA
B��l mol Fe(NO3)3������HI��Һ��Ӧʱת�Ƶĵ�����Ϊ3NA
C��1 mol Na2O2�����к���������Ϊ4NA
D�������£�1 mol Fe�����ŨHNO3��Ӧ��ת�Ƶ��ӵ���ĿΪ3 NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com