
�����ִ�����ҵ�ķ�չ����Դ�����Ѿ�Խ��Խ�������ǵ����ӣ���ѧ��Ԥ�ԣ�δ���������ȼ������ɫֲ�����ֲ��Ľոѣ���Ҫ�ɷ�����ά�أ������˵Ĵ�������ˮ��������ǣ��ٽ�������ת��Ϊ�Ҵ�������ȼ�ϣ�
��1��д����ɫֲ��Ľո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
������____________________________________��
������____________________________________��
��2���Ҵ�������ȼ���⣬���������ϳ������л��������Ҫ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ�����ڱ���������Ӧ���ʵĽṹ��ʽ��
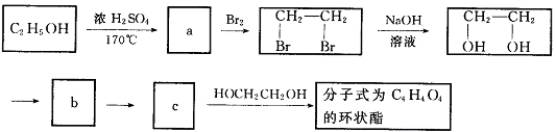
��3��д�������ϵͼ����![]() ��
��![]() �Ļ�ѧ����ʽ��
�Ļ�ѧ����ʽ��
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��1��д����ɫֲ��Ľո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
������____________________________________��
������____________________________________��
��2���Ҵ�������ȼ���⣬���������ϳ������л��������Ҫ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ�����ڱ���������Ӧ���ʵĽṹ��ʽ��

��3��д�������ϵͼ����![]() ��
��![]() �Ļ�ѧ����ʽ��
�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д����ɫֲ��ո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
��____________________����____________________��
(2)�Ҵ�������ȼ���⣬���������ϳ������л��������Ҫ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ�����ڱ���������Ӧ���ʵĽṹ��ʽ��

(3)д�������ϵͼ����CH2OHCH2OH��C4H4O4�Ļ�ѧ����ʽ��(�л����ýṹ��ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(C6H10O5)n+nH2O![]() nC6H12O6
nC6H12O6
C6H12O6![]()
(1)��֪��C2H5OH(l)+3O2(g)![]() 2CO2(g)+3H2O(l) ��H=-1 367 kJ��mol-1
2CO2(g)+3H2O(l) ��H=-1 367 kJ��mol-1
CH4(g)+2O2(g)![]() CO2(g)+2H2O(l) ��H=-890 kJ��mol-1
CO2(g)+2H2O(l) ��H=-890 kJ��mol-1
��ij��ֲ��ĽոѺ���ά��Լ50%����ֲ��ոѾ���һϵ��ת���õ��Ҵ�ԭ�ϵ���������Ϊ80%������1
(2)��ɫֲ�������õ�Ч����___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 3.3 ��ά�صĻ�ѧ�ӹ���ϰ���������棩 ���ͣ������
�����ִ�����ҵ�ķ�չ����Դ�����Ѿ�Խ��Խ�������ǵ����ӡ���ѧ��Ԥ�ԣ�δ���������ȼ������ɫֲ�����ֲ��Ľո�(��Ҫ�ɷ�����ά��)�����˵Ĵ�������ˮ��������ǣ��ٽ�������ת��Ϊ�Ҵ�������ȼ�ϡ�
(1)д����ɫֲ��ո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
��________________________________________________________________________��
��________________________________________________________________________��
(2)�Ҵ�������ȼ���⣬���������ϳ������л��������Ҫ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ��
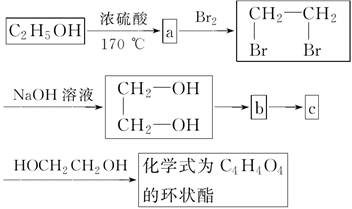
��д���������ʵĽṹ��ʽ
a��______________��b��______________��c��______________��
(3)д�������ϵͼ����c����C4H4O4�Ļ�ѧ����ʽ��(�л����ýṹ��ʽ��ʾ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com