
| A£® | pH=4.3µÄCH3COOHÓėCH3COONa»ģŗĻČÜŅŗÖŠ£ŗc£ØNa+£©£¼c£ØCH3COO-£© | |
| B£® | ÅضČĪŖ0.2mol/LµÄCH3COOHČÜŅŗŗĶÅ©µŲĪŖ0.1mol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗó£ŗc£ØCH3COO-£©-c£ØCH3COOH£©=2[c£ØH+£©-c£ØOH-£©] | |
| C£® | “×ĖįČÜŅŗ¼ÓÉŁĮæĖ®Ļ”ŹĶ£¬$\frac{c£ØC{H}_{3}COOH£©}{{c}^{2}£Ø{H}^{+}£©}$¼øŗõ²»±ä | |
| D£® | amol/LCH3COOHČÜŅŗÓėbmol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠc£ØNa+£©£¾c£ØCH3COO-£©£¬ŌņŅ»¶ØÓŠa”Üb |
·ÖĪö A”¢ČÜŅŗĻŌĖįŠŌ£¬¹Źc£ØH+£©£¾c£ØOH-£©£¬øł¾ŻµēŗÉŹŲŗ楓·ÖĪö£»
B”¢ÅضČĪŖ0.2mol/LµÄCH3COOHČÜŅŗŗĶÅضČĪŖ0.1mol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗóµĆÅØ¶Č¾łĪŖ0.05mol/LµÄCH3COOHŗĶCH3COONaµÄ»ģŗĻĪļ£¬øł¾ŻĪļĮĻŹŲŗćŗĶµēŗÉŹŲŗ楓·ÖĪö£»
C”¢“×ĖįČÜŅŗÖŠ¼ÓÉŁĮæĖ®Ļ”ŹĶ£¬µēĄėĘ½ŗā³£Źż²»±ä£¬¶ųČÜŅŗÖŠµÄc£ØH+£©=c£ØCH3COO-£©£»
D”¢amol/LCH3COOHČÜŅŗÓėbmol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠc£ØNa+£©£¾c£ØCH3COO-£©£¬Ōņøł¾ŻµēŗÉŹŲŗćæÉÖŖ£ŗc£ØH+£©£¼c£ØOH-£©£¬¾Ż“Ė·ÖĪö£®
½ā“š ½ā£ŗA”¢øł¾ŻµēŗÉŹŲŗćæÉÖŖ£ŗc£ØH+£©+c£ØNa+£©=c£ØOH-£©+c£ØCH3COO-£©£¬¶ųČÜŅŗĻŌĖįŠŌ£¬¼“c£ØH+£©£¾c£ØOH-£©£¬¹ŹæÉÖŖc£ØNa+£©£¼c£ØCH3COO-£©£¬¹ŹAÕżČ·£»
B”¢ÅضČĪŖ0.2mol/LµÄCH3COOHČÜŅŗŗĶÅضČĪŖ0.1mol/LµÄNaOHČÜŅŗµČĢå»ż»ģŗĻŗóµĆÅØ¶Č¾łĪŖ0.05mol/LµÄCH3COOHŗĶCH3COONaµÄ»ģŗĻĪļ£¬øł¾ŻĪļĮĻŹŲŗćæÉÖŖ£ŗ
c£ØCH3COOH£©+c£ØCH3COO-£©=2c£ØNa+£© ¢Ł
c£ØCH3COO-£©+c£ØOH-£©=c£ØNa+£©+c£ØH+£© ¢Ś
½«¢Ś”Į2-¢ŁæÉµĆ£ŗc£ØCH3COO-£©-c£ØCH3COOH£©=2[c£ØH+£©-c£ØOH-£©]£¬¹ŹBÕżČ·£»
C”¢“×ĖįČÜŅŗÖŠ¼ÓÉŁĮæĖ®Ļ”ŹĶ£¬µēĄėĘ½ŗā³£ŹżK=$\frac{c£Ø{H}^{+}£©•c£ØC{H}_{3}CO{O}^{-}£©}{c£ØC{H}_{3}COOH£©}$²»±ä£¬¶ųČÜŅŗÖŠµÄc£ØH+£©”Öc£ØCH3COO-£©£¬¹ŹK=$\frac{c£Ø{H}^{+}£©•c£ØC{H}_{3}CO{O}^{-}£©}{c£ØC{H}_{3}COOH£©}$”Ö$\frac{{c}^{2}£Ø{H}^{+}£©}{c£ØC{H}_{3}COOH£©}$¼øŗõ²»±ä£¬Ōņ$\frac{c£ØC{H}_{3}COOH£©}{{c}^{2}£Ø{H}^{+}£©}$¼øŗõ²»±ä£¬¹ŹCÕżČ·£»
D”¢amol/LCH3COOHČÜŅŗÓėbmol/LNaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠc£ØNa+£©£¾c£ØCH3COO-£©£¬Ōņøł¾ŻµēŗÉŹŲŗćæÉÖŖc£ØH+£©£¼c£ØOH-£©£¬¼“ČÜŅŗĻŌ¼īŠŌ£®µ±b=aŹ±£¬Į½ÕßĒ”ŗĆĶźČ«·“Ӧɜ³É“×ĖįÄĘ£¬ČÜŅŗĻŌ¼īŠŌ£»µ±b£¾aŹ±£¬NaOHČÜŅŗ¹żĮ棬ČÜŅŗĻŌ¼īŠŌ£»µ«µ±aÉŌĪ¢“óÓŚb¼““×ĖįÖ»ŹĒ¼«ÉŁĮæ¹żĮæŹ±£¬ČÜŅŗŅ²æÉŅŌĻŌ¼īŠŌ£¬¹Ź²»Ņ»¶ØÓŠa”Üb£¬¹ŹD“ķĪó£®
¹ŹŃ”D£®
µćĘĄ ±¾Ģāæ¼²éĮĖŃĪČÜŅŗÖŠµÄĄė×ÓÅØ¶ČµÄ“óŠ”±Č½ĻŅŌ¼°ÅŠ¶Ļ£¬øł¾ŻŃĪČÜŅŗÖŠµÄĪļĮĻŹŲŗć”¢µēŗÉŹŲŗćµČĪļÖŹµÄĮæÅØ¶Č¹ŲĻµĄ“ÅŠ¶Ļ£¬ĢāÄæÄѶČÖŠµČ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČĘų”¢¶žŃõ»ÆĮņ¶¼ÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬ĖüĆĒµÄĘÆ°×ŌĄķĻąĶ¬ | |
| B£® | “æ¾»µÄ¾§Ģå¹čŹĒĻÖ“ś¹āѧ¼°¹āĻĖÖĘĘ·µÄ»ł±¾ŌĮĻ | |
| C£® | Óƶ”“ļ¶ūŠ§Ó¦æɼų±šFeCl3ČÜŅŗŗĶFe£ØOH£©3½ŗĢå | |
| D£® | NH3µÄĖ®ČÜŅŗæÉŅŌµ¼µē£¬ĖłŅŌNH3ŹĒµē½āÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ĀČĘųĶØČėĻĀĮŠČÜŅŗÖŠ | ŹµŃéĻÖĻó | ½įĀŪ |
| A | µĪÓŠKSCNµÄFeCl2ČÜŅŗ | ±äŗģ | ĀČĘų¾ßÓŠ»¹ŌŠŌ |
| B | µĪÓŠ·ÓĢŖµÄNaOHČÜŅŗ | ĶŹÉ« | ĀČĘų¾ßÓŠĘư׊Ō |
| C | ×ĻÉ«ŹÆČļČÜŅŗ | ĻȱäŗģŗóĶŹÉ« | ĀČĘų¾ßÓŠĖįŠŌ”¢Ęư׊Ō |
| D | ÉŁĮæĀČĘųĶØČė“óĮæĖ®ÖŠ | ČÜŅŗpH£¼7 ³ŹĒ³»ĘĀĢÉ« | ĀČĘųÓėĖ®·“Ӧɜ³ÉĖįŠŌĪļÖŹ£¬ ĒŅøĆ·“Ó¦ĪŖæÉÄę·“Ó¦ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ¼×ѧɜÓĆ50mLĮæĶ²ĮæČ”46.70mLÅØŃĪĖį | |
| B£® | ŅŅѧɜÓĆ¹ć·ŗpHŹŌÖ½²ā¶ØČÜŅŗµÄĖį¼īŠŌ£ŗpH=14.5 | |
| C£® | ±ūѧɜÅäNaOHČÜŅŗ£¬ÓƵē×ÓĢģĘ½³ĘČ”¹ĢĢå1.220g | |
| D£® | ¶”ѧɜÓĆŗģ±śµĪ¶Ø¹ÜĮæČ”25.00mL0.1mol/LµÄŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 Ąė×ÓµÄĮłÖÖČÜŅŗ£¬ĻņĆæÖÖČÜŅŗÖŠ·Ö±š¼ÓČėÉŁĮæĒāŃõ»ÆÄĘ¹ĢĢå”¢ÉŁĮæÅØŃĪĖį£¬¼øµĪĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬ĘäĄė×ÓŹżÄ涼¼õÉŁµÄŹĒ£Ø””””£©
Ąė×ÓµÄĮłÖÖČÜŅŗ£¬ĻņĆæÖÖČÜŅŗÖŠ·Ö±š¼ÓČėÉŁĮæĒāŃõ»ÆÄĘ¹ĢĢå”¢ÉŁĮæÅØŃĪĖį£¬¼øµĪĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬ĘäĄė×ÓŹżÄ涼¼õÉŁµÄŹĒ£Ø””””£©| A£® | ¢Ł¢Ś¢Ü | B£® | ¢Ś¢Ū¢Ż | C£® | ¢Ł¢Ü | D£® | ¢Ū¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
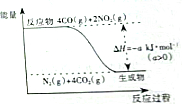 ŃŠ¾æNOx”¢SO2”¢COµČ“óĘųĪŪČ¾ĘųĢåµÄ“¦Ąķ·½·Ø¾ßÓŠÖŲŅŖŅāŅ壮
ŃŠ¾æNOx”¢SO2”¢COµČ“óĘųĪŪČ¾ĘųĢåµÄ“¦Ąķ·½·Ø¾ßÓŠÖŲŅŖŅāŅ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
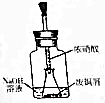 ĪŅ¹śĄśŹ·ÓĘ¾Ć£¬ĪÄĆ÷·¢“ļ£¬ŌēŌŚÉĢ“ś¾Ķ»įŅ±Į·ĶĘ÷£¬ŌŚ³öĶĮµÄø÷“śĶĘ÷±ķĆę¶¼ĪŖĀĢÉ«£®¾æĘѧ¼Ņ¼ģŃ飬ÕāŠ©ĶĘ÷¶¼ĪŖ¼īŹ½Ģ¼ĖįĶ[Cu2£ØOH£©2CO3]£®
ĪŅ¹śĄśŹ·ÓĘ¾Ć£¬ĪÄĆ÷·¢“ļ£¬ŌēŌŚÉĢ“ś¾Ķ»įŅ±Į·ĶĘ÷£¬ŌŚ³öĶĮµÄø÷“śĶĘ÷±ķĆę¶¼ĪŖĀĢÉ«£®¾æĘѧ¼Ņ¼ģŃ飬ÕāŠ©ĶĘ÷¶¼ĪŖ¼īŹ½Ģ¼ĖįĶ[Cu2£ØOH£©2CO3]£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪĀ¶Č/”ę | 1000 | 1100 |
| Ę½ŗā³£Źż | 0.68 | 0.50 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com