
����Ŀ���������繤ҵ���õķ�չ���˿ڵľ�����ȫ����Դ���ż�������������Խ��Խ���ص����⣬��ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�
(1)��ͼΪC����������ı仯��ϵͼ�����ٱ仯���û���Ӧ�����仯ѧ����ʽ������__________________����
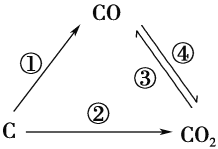
(2)��ú��Ϊȼ�Ͽ�ͨ����������;����
;�� ��C(s)��O2(g)===CO2(g)����H1��0����
;�� �������Ƴ�ˮú����C(s)��H2O(g)===CO(g)��H2(g)����H2��0����
��ȼ��ˮú����2CO(g)��O2(g)===2CO2(g)����H3��0����
2H2(g)��O2(g)===2H2O(g)����H4��0����
��;�� �� �ų�������__________(����������������������С����);�� �� �ų�����������H1����H2����H3����H4����ѧ��ϵʽ��____________________��
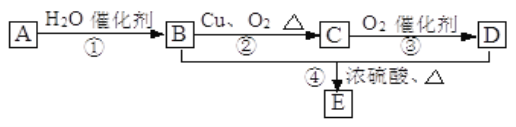
(3)�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ�Ͽ������·����ϳɼ״���
����һ��CO(g)��2H2(g)![]() CH3OH(g)
CH3OH(g)
��������CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)
CH3OH(g)��H2O(g)
��25 �桢101 kPa�£�1 g�״���ȫȼ�շ���22.68 kJ��д���״�ȼ���ȵ��Ȼ�ѧ����ʽ________________________________________________________________��
(4)���������ھ�������������ˮ������������ҵ�������ΪƯ�����������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)��O3(g)===3Ag2O(s)����H���D235.8 kJ��mol�D1 ��
��֪��2Ag2O(s)===4Ag(s)��O2(g)����H����62.2 kJ��mol�D1 ��
��O3ת��ΪO2���Ȼ�ѧ����ʽΪ__________________________��
���𰸡�C��CuO![]() Cu��CO��������H1����H2��1/2(��H3����H4)CH4O(l)��3/2O2(g)===CO2(g)��2H2O(l)����H����725.76 kJ��mol�D12O3(g)===3O2(g)����H����285 kJ��mol�D1
Cu��CO��������H1����H2��1/2(��H3����H4)CH4O(l)��3/2O2(g)===CO2(g)��2H2O(l)����H����725.76 kJ��mol�D12O3(g)===3O2(g)����H����285 kJ��mol�D1
��������
��1��C�ܽ�CuO�е�ͭ�û�������
��2�����ݸ�˹���ɿ�֪����Ӧ��ֻ��ʼ̬����̬�йأ����뷴Ӧ��;���أ��ɸ�˹���ɣ���;������������ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�ļӼ��������;��I���Ȼ�ѧ����ʽ���ݴ��ж���H1����H2����H3����H4����ѧ��ϵʽ��
��3���������������32g�״�ȼ�����ɶ�����̼��Һ̬ˮ���ȣ�����Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ���
��4����6Ag��s��+O3��g���T3Ag2O��s������H=-235.8kJmol-1����2Ag2O��s���T4Ag��s��+O2��g������H=+62.2kJmol-1�����ݸ�˹���ɿ�֪���2+����3�ɵõ���2O3��g���T3O2��g�����Դ˼��㷴Ӧ����
(1)�����û���Ӧ������̼��ˮ������Ӧ����һ����̼��������������������ﷴӦ����Ӧ�Ļ�ѧ����ʽΪC��CuO![]() Cu��CO������ȷ����C��CuO
Cu��CO������ȷ����C��CuO![]() Cu��CO����
Cu��CO����
(2)�ɸ�˹���ɿ�֪����һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ�����ݸ�˹���ɣ������ڣ��ۡ�1/2���ܡ�1/2��������H1����H2��1/2(��H3����H4)����ȷ������������H1����H2��1/2(��H3����H4)��
(3)��25 �桢101 kPa�£�1 g�״�(CH3OH)ȼ������CO2��Һ̬ˮʱ����22.68 kJ,32 g�״�ȼ�����ɶ�����̼��Һ̬ˮ�ų�����Ϊ725.76 kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH4O(l)��3/2O2(g)===CO2(g)��2H2O(l)����H����725.76 kJ��mol�D1����ȷ����CH4O(l)��3/2O2(g)===CO2(g)��2H2O(l)����H����725.76 kJ��mol�D1��
(4)��.6Ag(s)��O3(g)===3Ag2O(s)����H����235.8 kJ��mol�D1����.2Ag2O(s)===4Ag(s)��O2(g)����H����62.2 kJ��mol�D1�����ݸ�˹���ɿ�֪����2���ڡ�3�ɵõ���2O3(g)===3O2(g)����Ӧ����H��(��235.8 kJ��mol��1)��2��(��62.2 kJ��mol��1)��3����285 kJ��mol�D1����ȷ����2O3(g)===3O2(g)����H����285 kJ��mol�D1��


 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ȡ��������ϩʱ���������Ҵ���Ũ������ټ��ȵ�170������ȡ����ʵ�鳣���¶ȹ��߶���������Ӧ���в����Ҵ���ŨH2SO4��Ӧ����SO2��C02��ˮ������̿�ڡ�
I���÷���ʽ��ʾ��ϩ�Ʊ��ķ�Ӧԭ��____����ϩ�Ʊ�ʱ����140�泣�����и���Ӧ��������ѧ����ʽΪ_____��
�������������ʵ����ȷ�������������������ϩ�Ͷ�������������������⣺
��1��ͼ�Т٢ڢۢ�װ�ÿ�ʢ�ŵ��Լ��ǣ��Լ����ظ�ʹ�ã�Ҳ�ɲ�ʹ�ã�������дABCD��
��_____����____����_____����______��

A��Ʒ����Һ B��NaOH��Һ C��ŨH2SO4 D������KMnO4��Һ
��2����˵����������������ڵ�������______��
��3��ʹ��װ�âڵ�Ŀ����___________��
��4��ʹ��װ�â۵�Ŀ����______________��
��5��ȷ֤������ϩ��������_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ͻ���ϵ�������ʹ�����ҹ�������ǧ����ʷ���������ﲻ���ɺϽ���������( )
A.�ر���ٸB.Խ��������C.�����ұ���D.��̤������ͭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������CH4��C2H4��C2H6�����л��
(1)����������������������ȫȼ��ʱ��O2����������______�����ɵ�CO2����������______��
(2)ͬ״����ͬ�������������������ȫȼ��ʱ���ĵ�O2����������______��
(3)��120�棬1.01��105 Paʱ�������л����е����ֺ�������������ϵ�ȼ����ͬ�����²�÷�Ӧǰ���������û�з����仯��������������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��ͼ��ʾ����֪�л���A ��һ��ͬϵ��IJ������Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־����0.1mol A����������������ȫȼ�գ�����0.3 mol CO2��0.3 molˮ��B��D�����л��E�Ǿ���Ũ����ζ����������ˮ����״Һ�塣
��֪��-CHOH���ղ��ܱ�����Ϊ��COOH
(1)д��A�Ľṹ��ʽ_______��
(2)д��B�Ľṹ��ʽΪ_______��D�й����ŵ�����Ϊ_______��
(3)д�����з�Ӧ�����ͣ���_______����_______��
(4)д����������ת���Ļ�ѧ����ʽ�� B��C _________ ��B+D��E ______________��
��.��ʵ���ҿ�������ͼ��ʾ��װ�ý���B��D�ķ�Ӧ����ش��������⣺

(1)װ����ͨ�����ĵ���Ҫ���Թ���Һ����Ϸ�������Һ��������_______��
(2)��Ҫ���Ƶõ�E���������Ӧ���õ�ʵ�������_______��
��. ƻ������һ����ƻ�����Ͷ��ɵ�������Ʒ�����нⶾ����֬��ҩЧ��ƻ������ƻ������Ҫ�ɷ֣���ṹ��ʽ��ͼ��ʾ����ش��������⣺
![]()
(1)��һ�������£�ƻ���������������Щ���ʷ�����Ӧ��_______
A������������Һ B������ C��̼��������Һ D���Ҵ�
(2)0.1 molƻ���������������Ʒ�Ӧ�������ɱ�״���µ�����_______L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ũ���ݾ������أ���ũ���ٲݣ�������ʮ�������ò���⡣��Ҷ����Ԫ�صļ���ɾ������ĸ�������ɣ���������ѡ�õ�ʵ����Ʒ���ܶ��õ�����( )
A. ����Ҷ���ջһ���ѡ�â١��ں͢�
B. ��Ũ�����ܽ��Ҷ�Ҳ�������ˮϡ�ͣ�ѡ�âܡ��͢�
C. ���˵õ���Һ,ѡ�âܡ��ݺ͢�
D. ������Һ�е�Fe3+��ѡ�âۡ���͢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij���Na2CO3�������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣��Ҫʵ�鲽�����£�
�ٰ�ͼ��װ�����������װ�õ������ԣ�
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b g��
�ܴӷ�Һ©������6mol/L�����ᣬֱ�����ٲ�������ʱΪֹ��
�ݴӵ���A����������һ�����Ŀ�����
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g ��
���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g��
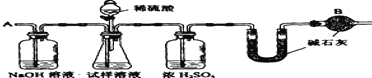
����պͻش����⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��______
����Ʒ�� ����Ʒ�� �������� ��������
��2��װ���и����B��������______
��3�������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ���� _____����ƫ�ߡ�ƫ�ͻ䣩�����ȱ��ʢŨ�����ϴ��ƿ�����ԵĽ����______����ƫ�ߡ�ƫ�ͻ䣩��
��4�������Ŀ����____________��
��5���������д�������������ļ���ʽΪ ________��������뻯��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ʒ�ˮ��ʹ��NaCN��������0.5 mg/L�����ɴﵽ�ŷű�����Ӧ���������С���һ��NaCN��NaClO��Ӧ������NaOCN��NaCl���ڶ���NaOCN��NaClO��Ӧ������Na2CO3��CO2��NaCl��N2����֪HCN�����ᣬ�ӷ����о綾��HCN��HOCN��NԪ�صĻ��ϼ���ͬ������˵����ȷ����
A. ����NaCN�����Ʒ�ˮ�Ĺ�������Ԫ�ػ��ϼ۵ĸı�
B. ��һ����Ӧ��ҺӦ����Ϊ���ԣ��ɱ��������ж�����HCN
C. �ڶ��������ķ�ӦΪ2OCN�� + 3ClO�� ![]() 2CO2�� + CO32 + 3Cl�� + N2��
2CO2�� + CO32 + 3Cl�� + N2��
D. ����100 m3��NaCN 10.3 mg/L�ķ�ˮʵ��������Ҫ50 mol NaClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������������ȷ����
A. ��״���£�1.12 L CCl4��������������ĿΪ3.7NA
B. ���³�ѹ�£�3.0 g�������ǵı������к��е�ԭ������Ϊ0.4NA
C. ����ʱ��56g Fe������Ũ���ᷴӦ��ת�Ƶĵ�����ĿΪ3NA
D. ����B�Ľṹ��Ԫ����ͼ ����11g����B����0.6NA�������Σ�����ԭ�ӹ��ɣ�
����11g����B����0.6NA�������Σ�����ԭ�ӹ��ɣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com