
ЁОЬтФПЁПНЊЛЦЫиЪЧвЛжжЬьШЛШОСЯЃЌЙЄвЕЩЯПЩгУЪЏгЭЕФСбНтВњЮяЭЈЙ§ШчЭМЗДгІжЦЕУЃК
вбжЊЃК![]()
![]() CH3CHO+
CH3CHO+![]()
![]()
![]() +H2O
+H2O
ЧыЛиД№ЯТСаЮЪЬтЃК
![]() ЪдМСXЮЊ ______ ЃЛ
ЪдМСXЮЊ ______ ЃЛ
![]() зюЖрФмЯћКФNaЁЂNaOHЁЂ
зюЖрФмЯћКФNaЁЂNaOHЁЂ![]() ЕФЮяжЪЕФСПЗжБ№ЮЊ3mo1ЁЂ2molЁЂ1molЃЌдђEЕФНсЙЙМђЪНЮЊ ______ ЃЛ
ЕФЮяжЪЕФСПЗжБ№ЮЊ3mo1ЁЂ2molЁЂ1molЃЌдђEЕФНсЙЙМђЪНЮЊ ______ ЃЛ
![]() НЊЛЦЫижаЕФКЌбѕЙйФмЭХГ§МзбѕЛљ
НЊЛЦЫижаЕФКЌбѕЙйФмЭХГ§МзбѕЛљ![]() ЭтЛЙга ______
ЭтЛЙга ______ ![]() аДУћГЦ
аДУћГЦ![]() ЃЛ
ЃЛ
![]() ЗДгІ
ЗДгІ![]() ЕФЛЏбЇЗНГЬЪНЮЊ ______ ЃЌЦфЗДгІРраЭЪЧ ______ ЃЛ
ЕФЛЏбЇЗНГЬЪНЮЊ ______ ЃЌЦфЗДгІРраЭЪЧ ______ ЃЛ
![]() ЗћКЯЯТСаЬѕМўGЕФЭЌЗжвьЙЙЬхЙВга ______ жжЃЌЦфжаКЫДХЙВеёЧтЦзжага5зщЗхЃЌЧвУцЛ§БШЮЊ2:2:2:1:1ЕФЪЧ ______ ЃЛ
ЗћКЯЯТСаЬѕМўGЕФЭЌЗжвьЙЙЬхЙВга ______ жжЃЌЦфжаКЫДХЙВеёЧтЦзжага5зщЗхЃЌЧвУцЛ§БШЮЊ2:2:2:1:1ЕФЪЧ ______ ЃЛ
ЂйЪєгкЗМЯуѕЅРр ЂкБНЛЗЩЯгаСНИіШЁДњЛљ ЂлФмгы![]() ШмвКЗЂЩњЯдЩЋЗДгІ
ШмвКЗЂЩњЯдЩЋЗДгІ
![]() НшМјжЦШЁНЊЛЦЫиЕФЗНЗЈвВФмКЯГЩШтЙ№ШЉ(
НшМјжЦШЁНЊЛЦЫиЕФЗНЗЈвВФмКЯГЩШтЙ№ШЉ(![]() )ЃЌаДГіжЦБИШтЙ№ШЉЫљашгаЛњЮяЕФНсЙЙМђЪН ______ ЁЃ
)ЃЌаДГіжЦБИШтЙ№ШЉЫљашгаЛњЮяЕФНсЙЙМђЪН ______ ЁЃ
ЁОД№АИЁПNaOHЕФЫЎШмвК 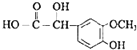 єЧЛљЁЂєЪЛљ
єЧЛљЁЂєЪЛљ ![]() бѕЛЏЗДгІ 9
бѕЛЏЗДгІ 9 ![]()
![]() ЁЂ
ЁЂ![]()
ЁОНтЮіЁП
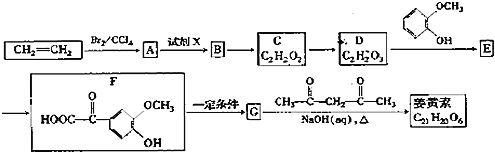
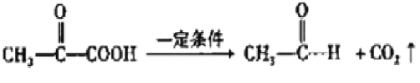
ввЯЉКЭфхЗЂЩњМгГЩЗДгІЩњГЩAЮЊBrCH2CH2BrЃЌгЩAЁњDЕФЯЕСазЊЛЏМАCЁЂDЗжзгЪНПЩжЊЃЌCжаШЉЛљбѕЛЏЩњГЩDЃЌдђAдкЧтбѕЛЏФЦЫЎШмвКЁЂМгШШЬѕМўЯТЗЂЩњЫЎНтЗДгІЩњГЩBЮЊHOCH2CH2OHЃЌBЗЂЩњДпЛЏбѕЛЏЩњГЩCЮЊOHC-CHOЃЌCжаВПЗжШЉЛљБЛбѕЛЏЩњГЩDЮЊOHC-COOHЃЌ1molEзюЖрФмЯћКФNaЁЂNaOHЁЂNaHCO3ЕФЮяжЪЕФСПЗжБ№ЮЊ3mo1ЁЂ2molЁЂ1molЃЌНсКЯFЕФНсЙЙПЩжЊЃЌDжаШЉЛљЗЂЩњМгГЩЩњГЩEЮЊ ЃЌEжаЕФШЉЛљБЛбѕЛЏЩњГЩFЃЌFдквЛЖЈЬѕМўЯТЗЂЩњаХЯЂiжаЭбєШЗДгІЩњГЩGЮЊ
ЃЌEжаЕФШЉЛљБЛбѕЛЏЩњГЩFЃЌFдквЛЖЈЬѕМўЯТЗЂЩњаХЯЂiжаЭбєШЗДгІЩњГЩGЮЊ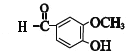 ЃЌНсКЯаХЯЂiiМАНЊЛЦЫиЕФЗжзгЪНЃЌПЩжЊНЊЛЦЫиЕФНсЙЙМђЪНЮЊЃК
ЃЌНсКЯаХЯЂiiМАНЊЛЦЫиЕФЗжзгЪНЃЌПЩжЊНЊЛЦЫиЕФНсЙЙМђЪНЮЊЃК ЁЃ
ЁЃ
(1)AЁњBЗЂЩњТБДњЬўЕФЫЎНтЗДгІЃЌЪдМСXЮЊNaOHЕФЫЎШмвКЃЌ
ЙЪД№АИЮЊЃКNaOHЕФЫЎШмвКЃЛ
(2)EЕФНсЙЙМђЪНЮЊЃК ЃЌ
ЃЌ
ЙЪД№АИЮЊЃК ЃЛ
ЃЛ
(3)НЊЛЦЫиЕФНсЙЙМђЪНЮЊЃК ЃЌНЊЛЦЫижаЕФКЌбѕЙйФмЭХГ§МзбѕЛљЃЈCH3OЃЉЭтЛЙгаЃКєЧЛљЁЂєЪЛљЃЌ
ЃЌНЊЛЦЫижаЕФКЌбѕЙйФмЭХГ§МзбѕЛљЃЈCH3OЃЉЭтЛЙгаЃКєЧЛљЁЂєЪЛљЃЌ
ЙЪД№АИЮЊЃКєЧЛљЁЂєЪЛљЃЛ
(4)ЗДгІBЁњCЕФЛЏбЇЗНГЬЪНЮЊЃК![]() ЃЌЪєгкбѕЛЏЗДгІЃЌ
ЃЌЪєгкбѕЛЏЗДгІЃЌ
ЙЪД№АИЮЊЃК![]() ЃЛбѕЛЏЗДгІЃЛ
ЃЛбѕЛЏЗДгІЃЛ
(5)G(
![]() ЕФЭЌЗжвьЙЙЬхЗћКЯЬѕМўЃКЂйЪєгкЗМЯузхѕЅРрЃЌЂкБНЛЗЩЯгаСНИіШЁДњЛљЃЌЂлФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЌЫЕУїКЌгаЗгєЧЛљЃЌЫљвдБНЛЗЩЯГ§ЗгєЧЛљжЎЭтЃЌСэвЛШЁДњЛљПЩФмЕФНсЙЙга-OOCCH3ЁЂ-COOCH3ЁЂ-CH2OOCHЃЌОљгаСкЁЂМфЁЂЖд3жжЃЌЫљвдЗћКЯЬѕМўЕФЯуРМШЉЕФЭЌЗжвьЙЙЬхЙВга9жжЃЌЦфжаКЫДХЙВеёЧтЦзжага5зщЗхЃЌЧвУцЛ§БШЮЊ2ЃК2ЃК2ЃК1ЃК1ЕФЪЧ
ЕФЭЌЗжвьЙЙЬхЗћКЯЬѕМўЃКЂйЪєгкЗМЯузхѕЅРрЃЌЂкБНЛЗЩЯгаСНИіШЁДњЛљЃЌЂлФмгыFeCl3ШмвКЗЂЩњЯдЩЋЗДгІЃЌЫЕУїКЌгаЗгєЧЛљЃЌЫљвдБНЛЗЩЯГ§ЗгєЧЛљжЎЭтЃЌСэвЛШЁДњЛљПЩФмЕФНсЙЙга-OOCCH3ЁЂ-COOCH3ЁЂ-CH2OOCHЃЌОљгаСкЁЂМфЁЂЖд3жжЃЌЫљвдЗћКЯЬѕМўЕФЯуРМШЉЕФЭЌЗжвьЙЙЬхЙВга9жжЃЌЦфжаКЫДХЙВеёЧтЦзжага5зщЗхЃЌЧвУцЛ§БШЮЊ2ЃК2ЃК2ЃК1ЃК1ЕФЪЧ![]() ЃЌ
ЃЌ
ЙЪД№АИЮЊЃК9ЃЛ![]() ЃЛ
ЃЛ
(6)НшМјжЦШЁНЊЛЦЫиЕФЗНЗЈвВФмКЯГЩШтЙ№ШЉ![]()
![]()
![]() ЃЌжЦБИШтЙ№ШЉЫљашгаЛњЮяЕФНсЙЙМђЪНЮЊ
ЃЌжЦБИШтЙ№ШЉЫљашгаЛњЮяЕФНсЙЙМђЪНЮЊ![]() ЁЂCH3CHOЃЌ
ЁЂCH3CHOЃЌ
ЙЪД№АИЮЊЃК![]() ЁЂCH3CHOЁЃ
ЁЂCH3CHOЁЃ


 ШЪАЎгЂгяЭЌВНСЗЯАВсЯЕСаД№АИ
ШЪАЎгЂгяЭЌВНСЗЯАВсЯЕСаД№АИ бЇЯАЪЕМљдАЕиЯЕСаД№АИ
бЇЯАЪЕМљдАЕиЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПH2SдкН№ЪєРызгЕФМјЖЈЗжЮіЁЂУКЛЏЙЄЕШСьгђЖМгаживЊгІгУЁЃЧыЛиД№ЃК
Ђё.ЙЄвЕЩЯвЛжжжЦБИH2SЕФЗНЗЈЪЧдкДпЛЏМСЁЂИпЮТЬѕМўЯТЃЌгУЬьШЛЦјгыSO2ЗДгІЃЌЭЌЪБЩњГЩСНжжФмВЮгыДѓЦјбЛЗЕФбѕЛЏЮяЁЃ
(1)ИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_____________ЁЃ
Ђђ.H2SПЩгУгкМьВтКЭГСЕэН№ЪєбєРызгЁЃ
(2)H2SЕФЕквЛВНЕчРыЗНГЬЪНЮЊ________ЁЃ
(3)вбжЊЃК25 ЁцЪБЃЌKsp(SnS)ЃН1.0ЁС10Ѓ25ЃЌKsp(CdS)ЃН8.0ЁС10Ѓ27ЁЃИУЮТЖШЯТЃЌЯђХЈЖШОљЮЊ0.1 molЁЄLЃ1ЕФCdCl2КЭSnCl2ЕФЛьКЯШмвКжаЭЈШыH2SЃЌЕБSn2ЃЋПЊЪМГСЕэЪБЃЌШмвКжаc(Cd2ЃЋ)ЃН________(ШмвКЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ
Ђѓ.H2SЪЧУКЛЏЙЄдСЯЦјЭбСђЙ§ГЬЕФживЊжаМфЬхЁЃЗДгІдРэЮЊ
ЂЁ.COS(g)ЃЋH2(g) ![]() H2S(g)ЃЋCO(g)ЁЁІЄHЃНЃЋ7 kJЁЄmolЃ1ЃЛ
H2S(g)ЃЋCO(g)ЁЁІЄHЃНЃЋ7 kJЁЄmolЃ1ЃЛ
ЂЂ.CO(g)ЃЋH2O(g) ![]() CO2(g)ЃЋH2(g)ЁЁІЄHЃНЃ42 kJЁЄmolЃ1ЁЃ
CO2(g)ЃЋH2(g)ЁЁІЄHЃНЃ42 kJЁЄmolЃ1ЁЃ
(4)вбжЊЃКЖЯСб1 molЗжзгжаЕФЛЏбЇМќЫљашЮќЪеЕФФмСПШчБэЫљЪОЁЃ
Зжзг | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
ФмСП/(kJЁЄmolЃ1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
БэжаxЃН________ЁЃ
(5)Яђ10 LШнЛ§ВЛБфЕФУмБеШнЦїжаГфШы1 mol COS(g)ЁЂ1 mol H2(g)КЭ1 mol H2O(g)ЃЌНјааЩЯЪіСНИіЗДгІЁЃЦфЫћЬѕМўВЛБфЪБЃЌЬхЯЕФкCOЕФЦНКтЬхЛ§ЗжЪ§гыЮТЖШ(T)ЕФЙиЯЕШчЭМЫљЪОЁЃ
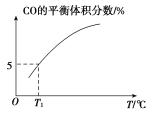
ЂйЫцзХЮТЖШЩ§ИпЃЌCOЕФЦНКтЬхЛ§ЗжЪ§_____(ЬюЁАдіДѓЁБЛђЁАМѕаЁЁБ)ЁЃдвђЮЊ_______
ЂкT1ЁцЪБЃЌВтЕУЦНКтЪБЬхЯЕжаCOSЕФЮяжЪЕФСПЮЊ0.80 molЁЃдђИУЮТЖШЯТЃЌCOSЕФЦНКтзЊЛЏТЪЮЊ_____ЃЛЗДгІЂЁЕФЦНКтГЃЪ§ЮЊ_____(БЃСєСНЮЛгааЇЪ§зж)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУвбжЊЮяжЪЕФСПХЈЖШЕФHClРДВтЖЈЮДжЊЮяжЪЕФСПХЈЖШЕФNaOHШмвКЪБЃЌбЁМзЛљГШзїжИЪОМСЃЌЪЙЫљВтЧтбѕЛЏФЦШмвКЕФХЈЖШЦЋЕЭЕФЪЧ( )
A.зЖаЮЦПгУеєСѓЫЎГхЯДКѓЮДгУД§ВтвКШѓЯД
B.ЖСШЁбЮЫсЬхЛ§ЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§
C.жеЕуЪБЃЌгавЛЕЮБъзМвКЙвдкЕЮЖЈЙмМтзьДІЮДЕЮШызЖаЮЦП
D.зАБъзМвКЕФЕЮЖЈЙмЕЮЖЈЧАМтзьДІгаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПXЁЂYЁЂZЁЂWЫФжждЊЫиЕФВПЗжаХЯЂШчЯТБэЫљЪОЁЃ
дЊЫи | X | Y | Z | W |
ЯрЙи аХЯЂ | ЖЬжмЦкдЊЫиЃЌзюИпЛЏКЯМлЮЊ+7Мл | ЛљЬЌдзгжаЃЌЕчзгеМОнЕФзюИпФмВуЗћКХЮЊLЃЌзюИпФмМЖЩЯжЛгаСНИізда§ЗНЯђЯрЭЌЕФЕчзг | КЫЭтЕчзгЙВга15жждЫЖЏзДЬЌ | ФмгыXаЮГЩСНжжГЃМћЛЏКЯЮяWX2ЁЂWX3ЃЌЗггіWX3ШмвКФмЗЂЩњЯдЩЋЗДгІ |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉWЕФЛљЬЌдзгЕчзгХХВМЪНЮЊ___ЃЌXЁЂYЁЂZШ§жждЊЫиЕчИКадгЩДѓЕНаЁЕФЫГађЮЊ___(гУОпЬхЕФдЊЫиЗћКХЬюаД)ЁЃ
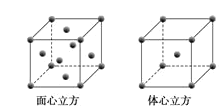
ЃЈ2ЃЉЛЏКЯЮяYX4ЁЂZX3ЁЂZX5(ЦјЬЌЛђвКЬЌЪБ)жаЃЌжааФдзгЕФЙьЕРРраЭВЛЪЧsp3дгЛЏЕФЪЧ___ (ЬюЛЏбЇЪНЃЌЯТЭЌЃЉЃЌЗжзгЙЙаЭЪЧе§ЫФУцЬхЕФЪЧ___ЃЌZX3Ъєгк___ЃЈМЋадЗжзгЁЂЗЧМЋадЗжзгЃЉЁЃ
ЃЈ3ЃЉвбжЊWX3ЕФШлЕуЃК306ЁцЃЌЗаЕуЃК319ЁцЃЌдђWX3ЕФОЇЬхРраЭЮЊ___ЁЃ
ЃЈ4ЃЉZдзгЕФМлЕчзгЙьЕРБэЪОЪНЮЊ___ЁЃ
ЃЈ5ЃЉWдЊЫиЕФЕЅжЪОЇЬхдкВЛЭЌЮТЖШЯТгаСНжжЖбЛ§ЗНЪНЃЌОЇАћЗжБ№ШчЭМЫљЪОЁЃдкУцаФСЂЗНОЇАћжаWдзгЕФХфЮЛЪ§ЮЊ___ЃЛШєWЕФдзгАыОЖЮЊrcmЃЌАЂЗќМгЕТТоГЃЪ§ЮЊNAЃЌдђЦфЬхаФСЂЗНОЇЬхЕФУмЖШПЩБэЪОЮЊ___gcm-3ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПБљОЇАћжаЫЎЗжзгЕФПеМфХХСаЗНЪНгыН№ИеЪЏОЇАћ(ЦфОЇАћНсЙЙШчЭМЃЌЦфжаПеаФЧђЫљЪОдзгЮЛгкСЂЗНЬхЕФЖЅЕуМАУцаФЃЌЪЕаФЧђЫљЪОдзгЮЛгкСЂЗНЬхФк)РрЫЦЁЃгаЙиБљОЇАћЕФЫЕЗЈКЯРэЕФЪЧ

A.БљОЇАћФкЫЎЗжзгМфвдЙВМлМќЯрНсКЯ
B.ОЇЬхБљгыН№ИеЪЏОЇЬхгВЖШЖМКмДѓ
C.БљЗжзгМфЕФЧтМќОпгаЗНЯђадКЭБЅКЭадЃЌвВЪЧвЛжжІвМќ
D.ЧтМќЕФДцдкЕМжТБљОЇАћгыН№ИеЪЏОЇАћЮЂСЃЕФХХСаЗНЪНРрЫЦ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЗДгІЃК2NO(g)ЃЋBr2(g)![]() 2NOBr(g) ЁїH=Ѓa kJЁЄmolЃ1 (a>0)ЃЌЦфЗДгІЛњРэШчЯТ
2NOBr(g) ЁїH=Ѓa kJЁЄmolЃ1 (a>0)ЃЌЦфЗДгІЛњРэШчЯТ
ЂйNO(g)ЃЋBr2(g)![]() NOBr2(g) Пь ЂкNO(g)ЃЋNOBr2(g)
NOBr2(g) Пь ЂкNO(g)ЃЋNOBr2(g)![]() 2NOBr(g) Т§
2NOBr(g) Т§
ЯТСагаЙиИУЗДгІЕФЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. ИУЗДгІЕФЫйТЪжївЊШЁОігкЂйЕФПьТ§
B. NOBr2ЪЧИУЗДгІЕФДпЛЏМС
C. е§ЗДгІЕФЛюЛЏФмБШФцЗДгІЕФЛюЛЏФмаЁa kJЁЄmolЃ1
D. діДѓBr2(g)ХЈЖШФмдіДѓЛюЛЏЗжзгАйЗжЪ§, МгПьЗДгІЫйТЪ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЭМЪОгыЖдгІЕФа№ЪіЯрЗћЕФЪЧ
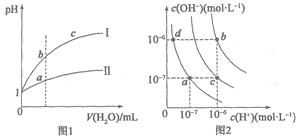
A.ЭМ1БэЪОЯрЭЌЮТЖШЯТpH=1ЕФбЮЫсКЭДзЫсШмвКЗжБ№МгЫЎЯЁЪЭЪБpHЕФБфЛЏЧњЯпЃЌЦфжаЧњЯпIIЮЊбЮЫсЃЌЧвbЕуШмвКЕФЕМЕчадБШaЕуЧП
B.ЭМ1жаЃЌжаКЭЕШЬхЛ§ЕФСНжжЫсЃЌЯћКФЕШХЈЖШЕФNaOHШмвКЬхЛ§VЃЈIЃЉЃОVЃЈIIЃЉ
C.ЭМ2жаДПЫЎНіЩ§ИпЮТЖШЃЌОЭПЩвдДгaЕуБфЕНcЕу
D.ЭМ2жадкbЕуЖдгІЮТЖШЯТЃЌНЋpH=2ЕФH2SO4гыpH=10ЕФNaOHШмвКЕШЬхЛ§ЛьКЯКѓЃЌШмвКЯджаад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДгКЃДјжаЬсШЁЕтЃЌПЩОЙ§вдЯТЪЕбщВНжшЭъГЩЁЃЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ( )

A.зЦЩеЙ§ГЬжаЪЙгУЕФВЃСЇвЧЦїгаОЦОЋЕЦЁЂЩеБЁЂВЃСЇАє
B.бѕЛЏЙ§ГЬжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ![]()
C.МьбщЕтЕЅжЪЪБЃЌПЩбЁгУЕэЗлЃЌШєЕэЗлБфРЖЫЕУїКЃДјжавЛЖЈКЌгаЕтЕЅжЪ
D.ЗжвКЪБЃЌЯШДђПЊЛюШћЗХГіЯТВувКЬхЃЌдйЙиБеЛюШћЃЌДгЩЯПкЕЙГіЩЯВувКЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃМћгаЛњЮяМфЕФзЊЛЏЙиЯЕШчЭМЫљЪО(вдЯТБфЛЏжаЃЌФГаЉЗДгІЬѕМўМАВњЮяЮДБъУї)ЁЃAЪЧЬьШЛгаЛњИпЗжзгЛЏКЯЮяЃЌDЪЧвЛжжживЊЕФЛЏЙЄдСЯЁЃдкЯрЭЌЬѕМўЯТЃЌGеєЦјУмЖШЪЧЧтЦјЕФ44БЖЁЃ
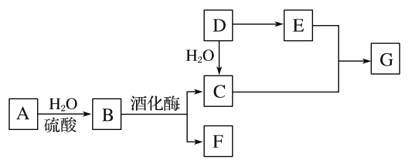
(1)DжаЙйФмЭХЕФУћГЦЃК______________________________________ЁЃ
(2)CКЭEЗДгІЕФЛЏбЇЗНГЬЪНЃК________________________ЁЃ
(3)МьбщAзЊЛЏЙ§ГЬжагаBЩњГЩЃЌЯШжаКЭЫЎНтвКЃЌдйашвЊМгШыЕФЪдМСЪЧ________________________________ЁЃ
(4)ФГЬўXЕФЯрЖдЗжзгжЪСПЪЧDЁЂFжЎКЭЃЌЗжзгжаЬМгыЧтЕФжЪСПжЎБШЪЧ5ЁУ1ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ________(ЬюзжФИ)ЁЃ
AЃЎX ВЛШмгкЫЎЃЌгыМзЭщЛЅЮЊЭЌЯЕЮя
BЃЎXаджЪЮШЖЈЃЌИпЮТЯТВЛЛсЗжНт
CЃЎXВЛДцдкКЌга3ИіМзЛљЕФЭЌЗжвьЙЙЬх
DЃЎXПЩФмКЭфхЫЎЗЂЩњМгГЩЗДгІ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com