


 ��
�� ��
�� ��
�� ���� ��C��D�����ķ�Ӧ�����Ϣ��֪��C����C=CH2�ṹ����ԭ��ΪCH3-CH��CH3��-CH3��A��BΪCH3-CCl��CH3��-CH3��CH3-CH��CH3��-CH2Cl��A��C������NaOH/����Һ�в����ȵ���ȥ��Ӧ��CΪCH3-C��CH3��=CH2��DΪCH3-CH��CH3��-CH2OH��E��������������ԭ�ӣ�FΪ���㻯��������Ϣ��֪��EΪHCHO����F�Ľṹ��֪��FΪ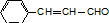 ��F��G����������Ӧ��GΪ����ϩ�ᣬD��G����������Ӧ����M����MΪ
��F��G����������Ӧ��GΪ����ϩ�ᣬD��G����������Ӧ����M����MΪ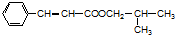 ��Ȼ�����л���Ľṹ�����������
��Ȼ�����л���Ľṹ�����������
��� �⣺��C��D�����ķ�Ӧ�����Ϣ��֪��C����C=CH2�ṹ����ԭ��ΪCH3-CH��CH3��-CH3��A��BΪCH3-CCl��CH3��-CH3��CH3-CH��CH3��-CH2Cl��A��C������NaOH/����Һ�в����ȵ���ȥ��Ӧ��CΪCH3-C��CH3��=CH2��DΪCH3-CH��CH3��-CH2OH��E��������������ԭ�ӣ�FΪ���㻯��������Ϣ��֪��EΪHCHO����F�Ľṹ��֪��FΪ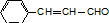 ��F��G����������Ӧ��GΪ����ϩ�ᣬD��G����������Ӧ����M����MΪ
��F��G����������Ӧ��GΪ����ϩ�ᣬD��G����������Ӧ����M����MΪ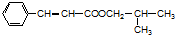 ��
��
��1��ԭ��C4H10ΪCH3-CH��CH3��-CH3��������2-�����飬�ʴ�Ϊ��2-�����飻
��2��F������Cu��OH��2��Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3��ͨ�����Ϸ���֪��N��M�Ľṹ��ʽ�ֱ�Ϊ



���� ���⿼���л���ĺϳɣ���ȷ�ϳ�·����̼���Ǽܵı仯�������Ļ�ѧ��Ӧ�Ƴ��������ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�


 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���ܵ���� | AgI | AgOH | Ag2S | PbI2 | Pb��OH��2 | PbS |
| Ksp | 8.3��10-12 | 5.6��10-8 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��10 mL����Ͳ��ȡ4.8 mL��Ũ���� | |
| B�� | ��������ʵ��ʱ��ʣ����ƷŻ�ԭ�Լ�ƿ | |
| C�� | ����FeCl3��Һʱ����FeCl3�������������У�Ȼ������ˮϡ�͵������Ũ�� | |
| D�� | ���Ʊ�Fe��OH��3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ˮ�����������͵�ԭ��������ˮ�����ڴ��������ɵ� | |
| B�� | F2��Cl2��Br2��I2������ɽṹ���ƣ���Է��������������Ӽ������������������۷е������� | |
| C�� | ����Ԫ�����ڱ���֪ʶ�ж�51Sb��һ�ֿ�����Ϊ�뵼����ϵķǽ���Ԫ�� | |
| D�� | ̼Ԫ���ж��ֺ��أ���12C��13C��14C��C60���ǻ�Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����ǿ��һ�־綾��ҩ����֪����ǿ�Ľṹ��ʽ��ͼ���йض���ǿ����ѧ�������Ǽ������İ������������˵����ȷ���ǣ�������
����ǿ��һ�־綾��ҩ����֪����ǿ�Ľṹ��ʽ��ͼ���йض���ǿ����ѧ�������Ǽ������İ������������˵����ȷ���ǣ�������| A�� | ����ǿ�������� | |
| B�� | ����ǿ������ | |
| C�� | ����ǿ�����ں�SO2����������ж��� | |
| D�� | ����ǿ�ķ���ʽΪC4H8N4S2O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���й����ʵ��������� | B�� | ��������ʵ������ĵ� | ||
| C�� | ��KMnO4��O2�����µĹ��� | D�� | մ��AgCl�������Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˹�����к����ļ�-CONH- | |
| B�� | ��˹����ˮ��ʱ���ɱ������ᡢ�춬����ͼ״� | |
| C�� | ��˹�������ܻ���Ӱ���ʧȥ��ζ�����а�˹�����ʳƷ���˼��� | |
| D�� | ��˹�����м���˫�����Լ�������õ��ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com