
ij��ѧ�о�С��ͨ���������ϵ�֪��ʵ���ҿ�ͨ������;���õ����������õ����ַ����ǣ�
�ٽ�����ͨ�����ȵ�����ͭ��ĩ���õ������ĵ�����ͭ��
�ڽ�����ͨ�����ȵ�ͭ���õ��ϴ����ĵ���������ͭ��ĩ��
�۽��������ƣ�NaNO2�����Ȼ�淋Ļ����Һ���ȣ��ݳ�������
��������ʵ��ʱ�ɴ�����ʵ������������̨�����С���Ȧ��ʯ�������ƾ��Ƶ�δ�г�����ѡ��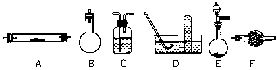
��ش��������⣺
��1��д���������з�����Ӧ�Ļ�ѧ����ʽ��_________________________��
��2�������������Ƶ�����Ӧ����_______�����ţ�װ���м���_______�������ʵĻ�ѧʽ�������������뷴Ӧ������Ӧ�пɹ۲쵽����Ҫ����Ϊ_________________________________��
��3����ȡ���������ַ����У������ٺͷ�����Խ��Խ�����ǵĹ�ע������������ʹ�á������ַ����뷽������ȣ�����Խ�����ڢ�____________________����_______________________________��������д����
 NaCl+N2��+2H2O
NaCl+N2��+2H2O


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 FeO+CO��+CO2��
FeO+CO��+CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���˱�ģ�� ���ͣ��ʴ���
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ�Һ���?Ī��ɣһ���ڻ�ѧԪ�ط���ʷ���������ش��ס�Ī��ɣ���������ѧ�о���������ȼ����������������ʯ����Ī��ɣ���������������������¼��Ȼ�ԭ���Ƶ�����ȼ����������ϸ�Ľ��������ڿ�����ײ�������Ȼ�ȼ�գ���
![]() ij�о���ѧϰС����ģ��Ī��ɣ��չ��ʵ�����Ʊ���ȼ���о�������û���ִ�����������������ϵ�֪����ͨ���ڸ������������¼��Ȳ���������FeC2O4���Ƶ����������� FeC2O4
ij�о���ѧϰС����ģ��Ī��ɣ��չ��ʵ�����Ʊ���ȼ���о�������û���ִ�����������������ϵ�֪����ͨ���ڸ������������¼��Ȳ���������FeC2O4���Ƶ����������� FeC2O4![]() FeO��CO����CO2��
FeO��CO����CO2��
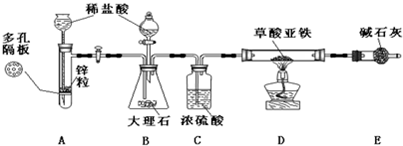
�ɴˣ�С�������һ�����Ʊ������������̺��Ʊ���ȼ����װ�ã�����̨�ȹ̶�����װ����ͼ����ȥδ������
![]()

��������װ�õ�ԭ���ش��������⣺
��1��д�������������Ʊ���ȼ���Ļ�ѧ����ʽ��
��2��������װ�ú�����Ҫ�����ǣ�
���������
��3��ʵ����Bװ�õ������ǣ� ��
��4�����еڶ����Ʊ���ȼ��ʵ�鲽�����ȷ˳���ǣ�
�ٵ�ȼD���ƾ��ƣ� �ڹر�A��B֮��Ļ������أ���Ϩ��ƾ��ƣ�����D�IJ�����ͨ����㹻ʱ��������ų�ԭ�������壬�ݼ���ͨ������D�IJ����ڹ�����ȴ�������������ߴ�A��B֮��Ļ�������
��5������װ����û�����Ե����⣿ ������,�ش���θĽ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���Ĵ�ʡ�˱��и߿���ѧ�����Ծ��������棩 ���ͣ������
 FeO+CO��+CO2��
FeO+CO��+CO2��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com