
 ��д����ӦX+Y�����Ȼ�ѧ����ʽ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��д����ӦX+Y�����Ȼ�ѧ����ʽ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
| ��������еijɷ� | K2AB3 | �� | ||
| ���ʵ���֮�� | ||||
���� ��1����Ԫ��λ�ڵ������ڵڢ����飬��Ԫ�ص���ɫ��Ӧ�Ǹ����ܲ���������ɫ��
��2����֪A���γɻ�������������Ԫ���ж�ΪC��B�ǵؿ��к�������Ԫ��ΪO��X��Y���ס����Ǻ�A��B��Ԫ�صĵ��ʻ������ת����ϵ��֪XΪC��YΪO2����ΪCO����ΪCO2����Ϊ���ۻ�����̼ԭ�Ӻ���ԭ��֮���γ�˫����ͼ�������֪1molX��1molY��Ӧ���ɼ�1mol��ʣ��Y0.5mol������Ȼ�ѧ����ʽ��д�Ȼ�ѧ����ʽ����ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ䣻
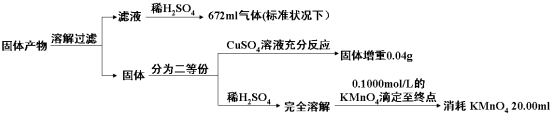
��3����Ԫ�ز���������ʽ���ڣ�����Ҳֻ��K2AB3ΪK2CO3����������ܽ���˵õ��������Һ����ҺΪ̼�����Һ������ϡ���ᷴӦ����672ml��״���µ�����Ϊ������̼�����ʵ���n��CO2��=$\frac{0.672L}{22.4L/mol}$=0.03mol�������Ϊ���ȷݣ���������ͭ��Һ��Ӧ��������0.04g��˵����������Fe+CuSO4=FeSO4+Cu����ϲ�������õ������ʵ����ʵ�������һ�ݼ���ϡ�����ܽ��ø��������Һ�ζ��յ�����20.00ml��Һ��˵�����������ӣ���ϻ�ѧ����ʽ������ϵ����õ����ж����ɲ������Ϊ��������������Ϊ̼��ء������������������Ԫ���غ�����ж����ɲ��
��4�����ݣ�3���ķ����жϺ�ԭ���غ���д��ѧ����ʽ��
��5��H2A2O4�Dz��ᣨH2C2O4���Ƕ�Ԫ���ᣬKHA2B4��Һ��KHC2O4��Һ�����ԣ���10mL 0.01mol•L-1��H2C2O4��Һ�μ�0.01mol•L-1KOH��ҺV��mL����
�ٵ�V��10mLʱ��H2C2O4��KOH��Ӧ����HC2O4 -�����в�����ࣻ
�ڵ�V=10mLʱ��H2C2O4��KOH��Ӧ����KHC2O4 -��KHC2O4��Һ������˵����Һ�е������ˮ�⣬�Ƚ���Һ��HC2O4-��C2O42-��H2C2O4��H+��Ũ�ȴӴ�С��˳��
�۵�V=amLʱ����Һ������Ũ�������¹�ϵ��c��K+��=2c��C2O42-��+c��HC2O4-����������Һ�е���غ����������Ũ�Ⱥ�����������Ũ����ͬ�жϣ�
�ܵ�V=bmLʱ����Һ������Ũ�������¹�ϵ��c��K+��=c��C2O42-��+c��HC2O4-��+c��H2C2O4������Һ�������غ����������ΪKHC2O4��
��� �⣺��1����Ԫ��λ�ڵ������ڵڢ����壬��Ԫ�ص���ɫ��Ӧ�Ǹ����ܲ���������ɫ����֤��������д��ڼ�Ԫ�صķ����ǣ�����ɫ��Ӧʵ�飬����ɫ�ܲ����۲�������ɫ����ɫ��
�ʴ�Ϊ���������ڵڢ����壬����ɫ��Ӧʵ�飬����ɫ�ܲ����۲�������ɫ����ɫ��
��2��������̼Ϊֱ���ͽṹ�������д�������̼��˫����������̼�ĵ���ʽΪ�� ��ͼ�������֪1molXhe 1molY��Ӧ���ɼ�1mol��ʣ��Y0.5mol����Ӧ����=393.5KJ/mol-282.9KJ/mol=110.6KJ/mol������Ȼ�ѧ����ʽ��д�Ȼ�ѧ����ʽ����ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ䣬��Ӧ���Ȼ�ѧ����ʽΪ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��ͼ�������֪1molXhe 1molY��Ӧ���ɼ�1mol��ʣ��Y0.5mol����Ӧ����=393.5KJ/mol-282.9KJ/mol=110.6KJ/mol������Ȼ�ѧ����ʽ��д�Ȼ�ѧ����ʽ����ע���ʾۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ䣬��Ӧ���Ȼ�ѧ����ʽΪ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
�ʴ�Ϊ�� ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��3����Ԫ�ز���������ʽ���ڣ�����Ҳֻ��K2AB3ΪK2CO3����������ܽ���˵õ��������Һ����ҺΪ̼�����Һ������ϡ���ᷴӦ����672ml��״���µ�����Ϊ������̼�����ʵ���n��CO2��=n��K2CO3��=$\frac{0.672L}{22.4L/mol}$=0.03mol�������Ϊ���ȷݣ���������ͭ��Һ��Ӧ��������0.04g��˵������������ӦΪ��Fe+CuSO4=FeSO4+Cu����ϲ�������õ������ʵ����ʵ�����
Fe+CuSO4=FeSO4+Cu��m
1 8
n 0.04g
n=0.005mol
����������0.01mol
��һ�ݼ���ϡ�����ܽ��ø��������Һ�ζ��յ�����20.00ml��Һ��˵�����������ӣ���ϻ�ѧ����ʽ������ϵ����õ����ж�KMnO4���仹ԭ����Ϊ��ɫMn2+��Һ����Һ��������Ӧ����ζ��յ������Ϊ���������һ�����Ը��������Һʱ��������ɫ�仯Ϊ�Ϻ�ɫ��������ڲ��仯��
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
5 1
n 0.02000L��0.1000mol/L
n=0.0100mol
��������������������Ϊ0.02mol���������������ʵ���Ϊ0.01mol
��K3Fe��C2O4��3•3H2O���ʵ���Ϊ0.02mol������̼���0.03mol������Fe���ʵ���Ϊ0.01mol������FeO���ʵ���Ϊ0.01mol���������ʵ���֮��Ϊ��
n��K2CO3����n��Fe����n��FeO��=0.03��0.01��0.01=3��1��1��
�ʴ�Ϊ�����������һ�����Ը��������Һʱ��������ɫ�仯Ϊ�Ϻ�ɫ��������ڲ��仯��
| ��������еijɷ� | K2AB3 | Fe | FeO | �� |
| ���ʵ���֮�� | 3��1��1 | |||
���� ���⿼����������ɡ����ʵ�̽�����̷����жϣ���Ҫ�Ƿ�Ӧ�����仯���Ȼ�ѧ����ʽ��д����ѧʽ����ɺͲ����жϣ��������Һ������Ũ�ȴ�С�Ƚϡ�����غ㡢�����غ��֪ʶ�㣬��Ϊ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������ | �Լ� | ������Ӧ�����ӷ���ʽ |
| A | K+��AlO2-��NO3- | ����CO2 | CO2+2H2O+AlO2-=Al��OH��3��+HCO3- |
| B | Fe3+��I-��ClO- | ����NaOH��Һ | Fe3++3OH-=Fe��OH�� 3�� |
| C | Ca2+��Na+��OH- | ����NaHCO3��Һ | HCO3-+OH-=CO32-+H2O |
| D | NH4+��HCO3-��Cl- | ����NaOH��Һ | NH4++OH-=NH3•H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
 ijԭ���װ����ͼ��ʾ������ܷ�ӦΪ2Ag+Cl2�T2AgCl�����ҺΪ1mol•L-1�����ᣮ����˵����ȷ���ǣ�������
ijԭ���װ����ͼ��ʾ������ܷ�ӦΪ2Ag+Cl2�T2AgCl�����ҺΪ1mol•L-1�����ᣮ����˵����ȷ���ǣ�������| A�� | ������ӦΪAgCl+e-�TAg+Cl- | |
| B�� | �ŵ�ʱ����Ĥ�Ҳ���Һ���д�����ɫ�������� | |
| C�� | �ŵ��Ĥ��Һ������ǿ | |
| D�� | ����·��ת��0.01 mol e-ʱ������Ĥ�����Һ��Լ����0.02mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�д������ֵ���ƽ�� | |
| B�� | ��ͬԭ��֮���γɵĹ��ۼ���һ���Ǽ��Թ��ۼ� | |
| C�� | ������ڿ����е�Na2SiO3��NaOH��CaCl2��Ư����Һ����������ᣬ���ܲ������� | |
| D�� | �������ữ��FeCl2��Һ�������������Һ�У���Һ��ƣ����������������ݣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH��5.6��7.0֮��Ľ�ˮͨ������Ϊ���� | |
| B�� | �������оƬ�����ά����ͨ���������ڹ����β�Ʒ | |
| C�� | ����β���еĵ������һ����̼��SO2�Ϳ�����������������Ⱦ���� | |
| D�� | ��ʳƷ���з���ʢ�й轺����ʯ�ҵ���С�����ɷ�ֹʳ���ܳ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
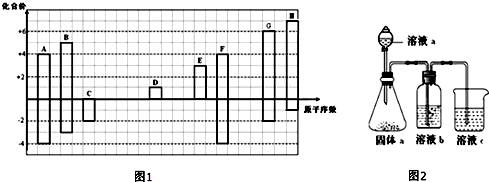
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
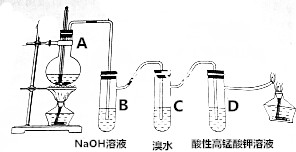
| A�� | ��ϩ�м������Ӽ����й��ۼ� | |
| B�� | װ��B�е����ӷ���ʽ��SO2+2OH-�TSO42-+H2O | |
| C�� | װ��C����ϩ����ȡ����Ӧ���������ᣬʹ��Һ��������ǿ | |
| D�� | װ��D����Һ����ɫ��ȥ����ϩ������������Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com