

| A�������ʾ��Һ�У���CH3COO-��+��OH-��=��CH3COOH�� +��H+�� |
| B�������ʾ��Һ�У���Na+��=��CH3COOH��+��CH3COO-�� |
| C�������ʾ��Һ�У���Na+�ݣ���OH���ݣ���CH3COO-��>��H+�� |
| D���ζ������п��ܳ��֣���CH3COOH�ݣ���CH3COO-�ݣ� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH4 ( g ) + 3/2O2 ( g ) = 2H2O ( l ) + CO ( g )��H1 |
| B��S ( s ) + 3/2O2 ( g ) = SO3 ( s )��H2 |
| C��C6H12O6 ( s ) + 6O2 ( g ) = 6CO2 (g) + 6H2O ( l )��H3 |
| D��CO ( g ) +1/2 O2 ( g ) = CO2 ( g )��H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.1mol��L-1 Na2S��Һ��һ���У�c��OH-���� c��H+��+ c��HS-����2c��H2S�� |
| B����NH4Cl��Һ�м���������ˮ���õ��ļ��Ի����Һ��һ���� �� c��Cl-����c��NH4+����c��OH-����c��H+�� |
| C������Na2S��Һ����Һc��OH-���϶�����c��H+���϶���С |
| D����ˮϡ��0.1mol��L-1������Һ������ƽ�������ƶ���c��H+��һ����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH = 3�������pH = 5������������ϣ�pH = 4 |
| B�������£�pH = 9�ļ�����Һ�У����ܴ���CH3COOH���� |
| C��������Һ�б�Ȼ��c(H+) = c(OH��) = 1��10��7 mol/L |
| D����0.1 mol/L HCl��Һ�м�������������ˮ����Һ�и������ӵ����ʵ���Ũ�Ⱦ���С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������¶� | B������ϡ���� |
| C����������� | D������ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
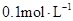 ijһԪ��
ijһԪ��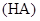 ��Һ��
��Һ��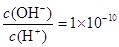 ������˵����ȷ����( )
������˵����ȷ����( )A����Һ����ˮ�������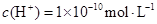 |
B����Һ��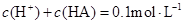 |
C����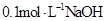 ��Һ�������Ϻ�������Һ�� ��Һ�������Ϻ�������Һ��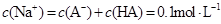 |
D��ԭ��Һ�м���һ����NaA������ˮϡ�ͣ���Һ��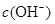 ������ ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
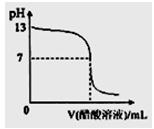
| A��pH=7ʱ�����Ӵ�����Һ�����Ϊ10mL |
| B��pH<7ʱ����Һ�У�c(CH3COO-)>c(Na+) |
| C��7<pH<13ʱ����Һ�У�c(Na+)>c(CH3COO-)>c(OH-)>c(H+) |
| D�������μ�0.1 mol��L��1������Һ����ҺpH���Ա�Ϊ1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Һ�ĵ���������a>b>c>d |
| B����Һ��pH��a>b>c>d |
| C��b��Һ�е�H+��Һ��d��Һ�е�OH-Ũ����� |
| D��c��Һ��d��Һ���ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��PH="7" | B��c��H+��=c��OH-�� |
| C��c��H+��=c��OH-��=10-7mol/L | D��c��H+����c��OH-��=10-14 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com