
����Ŀ����

��1��ʵ�����ܹ۲쵽��������____________________����ѡ����ţ�
A���Թ���þƬ���ܽ� B���Թ��в�����ɫ����
C���ձ���ڱ��� D���ձ��ײ�����������ɫ����
��2����ʵ����֪��MgCl2��Һ��H2��������________������ڡ���С�ڡ������ڡ���þƬ���������������
����50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��___________������֮���һ���������____________________��
��2�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ������ֵ��__________���ƫ��ƫС����Ӱ�족����
��1��úȼ�յķ�Ӧ�ȿ�ͨ����������;�������ã�a.����ú�ڳ���Ŀ�����ֱ��ȼ�ղ����ķ�Ӧ�ȣ�b.��ʹú��ˮ������Ӧ�õ�������һ����̼��Ȼ��ʹ�õ���������һ����̼�ڳ���Ŀ�����ȼ�ա����������̵��Ȼ�ѧ����ʽΪ��
a��C��s����O2��g��===CO2��g�� ��H��E1 ��
b��C��s����H2O��g��===CO��g����H2��g����H��E2 ��
H2��g����1/2O2��g��===H2O��g����H��E3 ��
CO��g����1/2O2��g��===CO2��g����H��E4 ��
�����E1��E2��E3��E4֮��Ĺ�ϵΪE2��_________________��
��2����ͼ��ʾ�ڳ��³�ѹ�£�1Ħ��NO2 ��1Ħ��CO��ȫ��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��___________________��

��3����ѧ��Ӧ���ʱ��뷴Ӧ���������ļ����йء���֪ijЩ��ѧ���ļ������±���ʾ��
���ۼ� | H��H | Cl��Cl | H��Cl |
����/��kJ��mol��1�� | 436 | 247 | 434 |
��Ӧ��H2��g��+Cl2��g��=2HCl��g�����ʱ䦤H �� ____________________��
���𰸡�A B DС�ڻ��β��������С�ձ��ںʹ��ձ���û��ƽ�루�������ʴ𰸸��֣�ƫСE2��E1��E3��E4NO2��g��+CO��g��=NO��g��+CO2��g�� ��H����234 kJ��mol��1��H ����185 kJ��mol��1
��������
��)(1)þ��������ҷ�Ӧ�������������ų��������ȣ������������Ƶ��ܽ�����¶����߶���С�����Ա���ʯ��ˮ���º���������������ʹ��Һ�ʻ���״��þ���ܽ⣬��ABDѡ���е�������ϣ��ʴ�Ϊ��ABD��
(2)����Ӧ����������������������ʱ����Ӧ�Ƿ��ȷ�Ӧ����MgCl2��Һ��H2��������С��þƬ����������������ʴ�Ϊ��С�ڣ�
��)(1)�����ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹����������ձ�Ϊһ���ߣ���������ɢʧ�ʴ�Ϊ�����β����������С�ձ��ںʹ��ձ���û��ƽ�룻
(2)���ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��)(1)��C(s)+O2(g)�TCO2(g)��H=E1����H2(g)+![]() O2(g)�TH2O(g)��H=E3����CO(g)+
O2(g)�TH2O(g)��H=E3����CO(g)+![]() O2(g)�TCO2(g)��H=E4�����ݸ�˹���ɣ���-��-�ܿɵã�C(s)+H2O(g)�TCO(g)+H2(g)����E2=E1-E3-E4���ʴ�Ϊ��E1-E3-E4��
O2(g)�TCO2(g)��H=E4�����ݸ�˹���ɣ���-��-�ܿɵã�C(s)+H2O(g)�TCO(g)+H2(g)����E2=E1-E3-E4���ʴ�Ϊ��E1-E3-E4��
(2)��ͼ��֪��1Ħ��NO2��1Ħ��CO��ȫ��Ӧ����CO2��NO�ų�����Ϊ(368-134)kJ=234kJ����Ӧ�Ȼ�ѧ����ʽΪ��NO2(g)+CO(g)�TNO(g)+CO2(g)��H=-234 kJmol-1���ʴ�Ϊ��NO2(g)+CO(g)�TNO(g)+CO2(g)��H=-234 kJmol-1��
(3)��Ӧ��=��Ӧ���ܼ���-�������ܼ��ܣ��ʷ�Ӧ��H2(g)+Cl2(g)�T2HCl(g)���ʱ���H=436kJ/mol+247kJ/mol-2��434kJ/mol=-185kJ/mol���ʴ�Ϊ��-185 kJ/mol��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. �����ܽ�ﵽƽ��ʱ����Һ�����ʵ�����Ũ����ȣ��ұ��ֲ���
B. ��֪BaSO4�ܶȻ�����1.0��10��10����BaSO4��ˮ���ܽ��Ϊ2.33��10��4g/100gˮ
C. Ksp(AgCl)��1.8��10��10��Ksp(AgI)��1.0��10��16,��������AgCl��Ҫ��NaI��Һ�п�ʼת��ΪAgI����NaI��Ũ��һ����![]() ��10��11mol��L��1
��10��11mol��L��1
D. ����MgCO3�������Һ�еμ�����Ũ����(��������仯),��Һ�е�c(Mg2+)��С��Ksp(MgCO3) ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ���Ƿ���Cl-��ѡ�õ��Լ���ϡ�����AgNO3��Һ������ϡ����������ǣ� ��
A.���ٷ�Ӧ�Ľ���B.�ų�ijЩ�������ӵĸ���
C.���ɲ�����ˮ������D.�Ӵ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л������ʵ�˵����ȷ���� �� ��
A. ��ϩ�ͼ��鶼������������Ӧ B. ��ϩ�;���ϩ����ʹ��ˮ��ɫ
C. ��ϩ�ͱ�����ʹ���Ը��������Һ��ɫ D. ���������Ʋ������������Ҵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ������ȤС��Ϊ̽������Һ��֮��ķ�Ӧ������ʵ����װ����ͼ��ʾ�������Ҫ��ش�������⡣(��֪���ķе�Ϊ80.1 �����ܶ�Ϊ0.9 g/mL )

��1��д������Һ�巴Ӧ�Ļ�ѧ����ʽ_____________________����Ӧ����Ϊ___________
��2����ƿ���е���ɫ�������ɣ�����ͬѧ�������ۺ���Ϊ�����ݸ�������ȷ�����������Ϸ�Ӧ�������ǣ�______________________________________������б�Ҫ��ʵ����иĽ���
��3�������������ʵ������ͼѡȡ���ʵ�װ�ú��Լ�������ʵ����иĽ���
![]()
I _____________������ĸ����ͬ��, II _______________
A��װ��NaOH��Һ��ϴ��ƿ B��װ��CC14��ϴ��ƿ
C��װ��KI��Һ��ϴ��ƿ D��װ��ʪ�����KI��ֽ�ļ���ƿ
��С��ͬѧ�ԸĽ�ʵ����Bװ���в����ĵ���ɫ���������й��ˡ�ϴ�ӡ���������������ϲ����У�ϴ�ӳ����IJ���Ϊ___________________________________________________
����ʵ����ȡ�õı�Ϊ17.3 mL��Һ���Թ��������ղ�õij�������Ϊ18.8 g�����ڸ÷�Ӧ�е�ת����Ϊ ____________��������λ��Ч���֣�������ͬѧ��Ϊת���ʹ��ͣ����˿��ܷ�������Ӧ�ͷ�Ӧ���ܽ��в���ȫ�⣬����������ܵ�ԭ����__________________________��__________________������2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������CN���ķ�ˮ�����У�Һ���ڼ��������¿��Խ��軯��������������(�䶾�Խ�Ϊ�軯���ǧ��֮һ)�������ν�һ��������Ϊ�����ʡ�
��1��ij����ˮ�к�KCN����Ũ��Ϊ650mg/L���������������������������·�Ӧ(����N��Ϊ-3��)��KCN��2KOH��Cl2��KOCN��2KCl��H2O����������Ԫ����_______��
��2��Ͷ�����Һ�ȣ��ɽ������ν�һ������Ϊ����������ƽ���л�ѧ����ʽ�����������ת�Ʒ������Ŀ_______________________________��
KOCN��KOH��Cl2��CO2��N2��KCl��H2O
��3��������������ˮ100L��ʹKCN��ȫת��Ϊ�����ʣ�������Һ��_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��F����ѧ��ѧ�г������ʣ�����A��C��EΪ���壬��A��ʹƷ����Һ��ɫ��B��DΪҺ�壬D��Ũ��Һ�ڳ�������ʹ���ۻ���F��Ũ��Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӡ�(��Ӧ�в�������������ȥ��
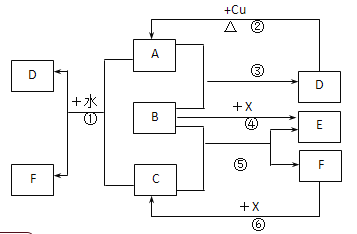
(1)д����Ӧ�ڵĻ�ѧ����ʽ��___________________��
(2)д����Ӧ�١��ݵ����ӷ���ʽ����____________����________________��
(3)����ͼ����Ϣ��B��C��D��X�����Դ�ǿ������˳����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯������������;��������ʵ������������±���ʾ��

�ش��������⣺
(1)��̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼ(�������ʽ)Ϊ____________________________����̬Sԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ________�Ρ�
(2)���ݼ۲���ӶԻ������ۣ�H2S��SO2��SO3����̬�����У�����ԭ�Ӽ۲���Ӷ�����ͬ���������ӵ���__��
(3)ͼ(a)ΪS8�Ľṹ�����۵�ͷе�Ҫ�ȶ���������۵�ͷе�ߺܶ࣬��Ҫԭ��Ϊ__________________��

(4)��̬���������Ե�������ʽ���ڣ�����ӵ����幹��Ϊ________�Σ����й��ۼ���������________�֣��������������д�����ͼ(b)��ʾ�����۷��ӣ��÷�����Sԭ�ӵ��ӻ��������Ϊ________��
(5)FeS2����ľ�����ͼ(c)��ʾ�������߳�Ϊanm��FeS2���ʽ��ΪM�������ӵ�������ֵΪNA���侧���ܶȵļ������ʽΪ______________________________g��cm��3��������Fe2��λ��![]() ���γɵ�������������ģ�����������ı߳�Ϊ________nm��
���γɵ�������������ģ�����������ı߳�Ϊ________nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ������������ʵ�鷽����
��1������һ����ȡһ����������Ʒ(30g)�����������м��������أ���ȴ����ȡʣ���������Ϊ31.6g�����㡣��ʵ���м��������ص�Ŀ����_________________________������Ʒ��Na2CO3����������Ϊ_______________________________
��2��������������ͼ��ʾװ�ý���ʵ�飺������̨�����е�����δ��ͼ�л�����
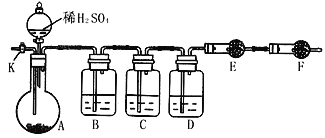
����֪����C��װ��Ʒ����Һ����������______________�������������Һ��������Na2CO3�����IJ��������ʵ��ֵƫ�ͣ�������____________________��
��ʵ�����б������³����Լ���a.Ũ���� b.Ʒ����Һ c.���Ը��������Һd.����������Һ e.��ˮ����ͭ f.��ʯ�� g.���������� h.��ˮ�Ȼ��� �뽫����������Ӧʢ�ŵ��Լ����������Ӧ�ո�B��______��D��________��E��________��
��ʵ������У�������A�ڵĹ��巴Ӧ��ȫ�������K����A��ͨ������ĵ�������������Ŀ����______________��
��3������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м�������BaCl2��Һ�����ˡ�ϴ�ӡ������������������������ʵ�����жϳ���ϴ�ɾ��ķ�����_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com