
��12�֣�����״����һ�������CO2����ͨ�����ΪV����NaOH��Һ�У���ַ�Ӧ���ڼ�ѹ���µ�������������Һ���õ���ɫ����
��1������CO2ͨ������ͬ�����õ��İ�ɫ��������Ҳ��ͬ���ƶϲ�д�����ֿ�����ɵĻ�ѧʽ�����Բ�������Ҳ����������ţ�
�� ���� ���� ���� ��
��2������Ӧ���Ⱥ�˳��д��������Ӧ�����ӷ���ʽ��
��
��3������Ӧ��CO2��NaOH����ʣ�࣬��Ӧ������Һ�м�������ij���ʯ��ˮ����a�˰�ɫ����
�ٸ����������ݣ��ܷ���������������ͨ���CO2����������ܣ��ô���ʽ��ʾ�����V��CO2��= �����ܣ������� ��
�ڸ����������ݣ��ܷ����������NaOH��Һ��Ũ�ȣ����ܣ��ô���ʽ��ʾ��Ũ��C��NaOH��= �����ܣ������� ��
��1����NaOH��Na2CO3 ��1�֣�����Na2CO3��1�֣�����Na2CO3��NaHCO3��1�֣�����NaHCO3��1�֣�
��2��2OH��+CO2= CO32-+ H2O��2�֣������� CO32-+ H2O +CO2= 2HCO3��2�֣�
��3����V��CO2��=0.224a L��2�֣�
�ڵ���Ӧ���������ֻ��NaHCO3ʱ����n(NaOH)=n(NaHCO3)������Ӧ���������ֻ��Na2CO3ʱ,��n(NaOH)=2n(Na2CO3)������Ӧ�����������Na2CO3��NaHCO3ʱ�����ĵ�NaOH�������Ķ���֮�䣬����NaOH��Һ�����ʵ���Ũ�Ȳ���ȷ������2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣� CO2��NaOH�ķ�Ӧ��һ���ܻ����Ļ�ѧ��Ӧ���кܶ��ʵ�鷽������ͨ���۲쵽����������˵��CO2��NaOH��Һ�����˷�Ӧ�����ṩ����ʵ����Ʒ������ƿ����ƿ����Һ©��������©�������ܡ���Ƥ�ܡ����ɼС��ձ�����Ͳ��ˮ��CO2���塢NaOH��Һ�Լ�����Ϊ�����õ���������ҩƷ��������λͬѧ�������ͼ��ʾ��A��E���װ�ã��Իش�
(1)��ͼA��������Һ©���е�NaOH��Һ������ƿʱ���������ˮ���е�ˮ�����뵽��ƿ�У���֤��CO2��NaOH��Һ�����˷�Ӧ����д���˹�����NaOH��Һ��CO2���ܷ����ķ�Ӧ���ӷ���ʽ��__________________��____________________��
(2)��ͼB��E����ָ���ܴﵽʵ��Ŀ�ĵ�װ��______________����B��C��D��E��գ���B�г���_______________________�����֤��CO2��NaOH�����˷�Ӧ��
(3)����״����һ�������CO2���建��ͨ��V L NaOH��Һ�У����CO2��NaOH����ʣ�࣮�ڷ�Ӧ�����Һ�м��������ij���ʯ��ˮ�õ�W g�����������������ܷ�ȷ��CO2����������ܣ����������������CO2������������ܣ�������ʲôʵ�飿��Ҫ˵��������ʵ���õ�������ʽ��ʾ��_________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�������ʡ����ʵ����ѧ��һ��ѧ�ڿ�ѧ���Ի�ѧ�Ծ� ���ͣ�������
����״����һ�������ij���壨Ħ������ΪM g/mol������a gˮ�У���ѧ��Ӧ�������õ�����Һ�ܶ�Ϊb g/ml�����ʵ���Ũ��Ϊc mol/L��������������� L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����е���ʮһ��ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
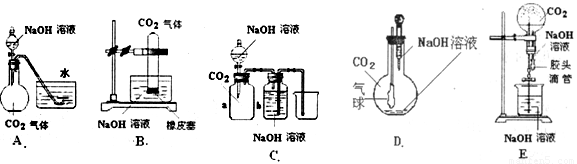
��10�֣� CO2��NaOH�ķ�Ӧ��һ���ܻ����Ļ�ѧ��Ӧ���кܶ��ʵ�鷽������ͨ���۲쵽����������˵��CO2��NaOH��Һ�����˷�Ӧ�����ṩ����ʵ����Ʒ������ƿ����ƿ����Һ©��������©�������ܡ���Ƥ�ܡ����ɼС��ձ�����Ͳ��ˮ��CO2���塢NaOH��Һ�Լ�����Ϊ�����õ���������ҩƷ��������λͬѧ�������ͼ��ʾ��A��E���װ�ã��Իش�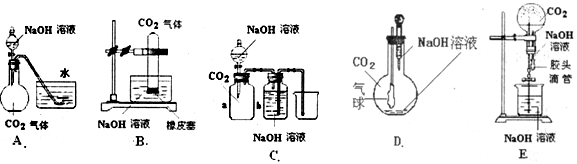
(1)��ͼA��������Һ©���е�NaOH��Һ������ƿʱ���������ˮ���е�ˮ�����뵽��ƿ�У���֤��CO2��NaOH��Һ�����˷�Ӧ����д���˹�����NaOH��Һ��CO2���ܷ����ķ�Ӧ���ӷ���ʽ��__________________��____________________��
(2)��ͼB��E����ָ���ܴﵽʵ��Ŀ�ĵ�װ��______________����B��C��D��E��գ���B�г���_______________________�����֤��CO2��NaOH�����˷�Ӧ��
(3)����״����һ�������CO2���建��ͨ��V L NaOH��Һ�У����CO2��NaOH����ʣ�࣮�ڷ�Ӧ�����Һ�м��������ij���ʯ��ˮ�õ�W g�����������������ܷ�ȷ��CO2����������ܣ����������������CO2������������ܣ�������ʲôʵ�飿��Ҫ˵��������ʵ���õ�������ʽ��ʾ��_________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣� CO2��NaOH�ķ�Ӧ��һ���ܻ����Ļ�ѧ��Ӧ���кܶ��ʵ�鷽������ͨ���۲쵽����������˵��CO2��NaOH��Һ�����˷�Ӧ�����ṩ����ʵ����Ʒ������ƿ����ƿ����Һ©��������©�������ܡ���Ƥ�ܡ����ɼС��ձ�����Ͳ��ˮ��CO2���塢NaOH��Һ�Լ�����Ϊ�����õ���������ҩƷ��������λͬѧ�������ͼ��ʾ��A��E���װ�ã��Իش�
(1)��ͼA��������Һ©���е�NaOH��Һ������ƿʱ���������ˮ���е�ˮ�����뵽��ƿ�У���֤��CO2��NaOH��Һ�����˷�Ӧ����д���˹�����NaOH��Һ��CO2���ܷ����ķ�Ӧ���ӷ���ʽ��__________________��____________________��
(2)��ͼB��E����ָ���ܴﵽʵ��Ŀ�ĵ�װ��______________����B��C��D��E��գ���B�г���_______________________�����֤��CO2��NaOH�����˷�Ӧ��
(3)����״����һ�������CO2���建��ͨ��V L NaOH��Һ�У����CO2��NaOH����ʣ�࣮�ڷ�Ӧ�����Һ�м��������ij���ʯ��ˮ�õ�W g�����������������ܷ�ȷ��CO2����������ܣ����������������CO2������������ܣ�������ʲôʵ�飿��Ҫ˵��������ʵ���õ�������ʽ��ʾ��_________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com