·ÖĪö£ŗ£Ø1£©øł¾Żn=cv¼ĘĖćČÜÖŹNa
2CO
3µÄĪļÖŹµÄĮ棬ĄūÓĆNa
2CO
3?10H
2OµÄĪļÖŹµÄĮæµČÓŚNa
2CO
3µÄĪļÖŹµÄĮ棬øł¾Żm=nM¼ĘĖćNa
2CO
3?10H
2OµÄÖŹĮ棻øł¾Żc=
·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæ»ņ¶ŌČÜŅŗµÄĢå»żµÄÓ°ĻģÅŠ¶Ļ£»
£Ø2£©øł¾ŻŹµŃé²Ł×÷µÄ²½ÖčŅŌ¼°Ćæ²½²Ł×÷ŠčŅŖŅĒĘ÷Č·¶Ø·“Ó¦ĖłŠčŅĒĘ÷£»
£Ø3£©ÓĆ½ŗĶ·µĪ¹ÜµĪ¼ÓČÜŅŗµÄ²Ł×÷Ćū³ĘŹĒ¶ØČŻ£»ÓĆÕōĮóĖ®Ļ“µÓ²£Į§°ōµÄ²Ł×÷Ćū³ĘŹĒĻ“µÓ£»øł¾Żc=
·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæ»ņ¶ŌČÜŅŗµÄĢå»żµÄÓ°ĻģÅŠ¶Ļ£»
£Ø4£©øł¾ŻÅäÖĘČÜŅŗµÄŹµŃé²Ł×÷¹ż³Ģ½ųŠŠŹµŃé²½ÖčÅÅŠņ£®
½ā“š£ŗ½ā£ŗ£Ø1£©ŹµŃéŹŅÅäÖĘ100mL 0.100mol?L
-1 Na
2CO
3ČÜŅŗŠčŅŖNa
2CO
3µÄĪļÖŹµÄĮæĪŖ£ŗ0.1L”Į0.1mol/L=0.01mol£¬Na
2CO
3?10H
2OµÄĪļÖŹµÄĮæĪŖ0.01mol£¬Na
2CO
3?10H
2OµÄÖŹĮæĪŖ£ŗ0.01mol”Į286g/mol=2.86g£¬µ«ĢģĘ½µÄ¾«Č·¶ČĪŖ0.1g£¬ĖłŅŌŠčŅŖ³ĘĮ澧ĢåĪŖ2.9g£»ČōĖłČ”µÄ¾§ĢåŅŃ¾ÓŠŅ»²æ·ÖŹ§Č„ĮĖ½į¾§Ė®£¬ČÜÖŹĢ¼ĖįÄʵÄÖŹĮæĘ«“󣬼“nĘ«“ó£¬øł¾Żc=
·ÖĪöæÉÖŖČÜŅŗµÄÅضČĘ«“ó£¬
“š°øĪŖ£ŗ2.9£»“ó£»
£Ø2£©ÅäÖĘ²½ÖčÓŠ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬Ņ»°ćÓĆĶŠÅĢĢģĘ½³ĘĮ棬ÓĆŅ©³×Č”ÓĆŅ©Ę·£¬ŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó×ŖŅʵ½100mLČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬ĖłŅŌŠčŅŖµÄŅĒĘ÷ĪŖ£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢100mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬
¹Ź“š°øĪŖ£ŗ100mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü”¢²£Į§°ō£»
£Ø3£©¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬ÓĆ½ŗĶ·µĪ¹ÜĻņČŻĮæĘæÖŠµĪ¼ÓČÜŅŗ£¬øĆ²Ł×÷µÄĆū³ĘŹĒ¶ØČŻ£»
Čē¹ūø©ŹÓæĢ¶ČĻߣ¬ČÜŅŗµÄĢå»żĘ«Š”£¬øł¾Żc=
·ÖĪöæÉÖŖÅäÖʵÄÅØ¶Č½«Ę«“ó£»
×ŖŅĘŗó£¬ÓĆÕōĮóĖ®Ļ“µÓ²£Į§°ōµÄ²Ł×÷Ćū³ĘŹĒĻ“µÓ£»
ČōƻӊĻ“µÓ£¬ČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬øł¾Żc=
·ÖĪöæÉÖŖÅäÖʵÄÅØ¶Č½«Ę«Š”£»
¹Ź“š°øĪŖ£ŗ¶ØČŻ£»“ó£»Ļ“µÓ£»Š”£»
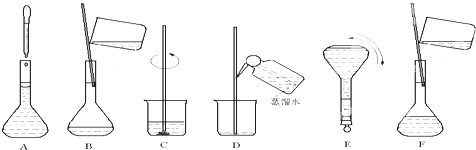
£Ø4£©²Ł×÷²½ÖčÓŠ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓŅĘŅŗ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬ĖłŅŌŹµŃé¹ż³ĢĻČŗó“ĪŠņÅÅĮŠĪŖ£ŗC”¢B”¢D”¢F”¢A”¢E£¬
¹Ź“š°øĪŖ£ŗC”¢B”¢D”¢F”¢A”¢E£»




 ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø