
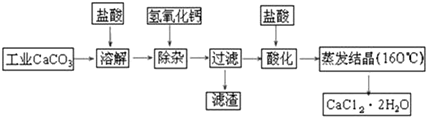
·ÖĪö ¹¤ŅµĢ¼ĖįøĘ¼ÓČėŃĪĖįæÉĶźČ«ČܽāÉś³ÉĀČ»ÆøĘ”¢ĀČ»ÆĢśŅŌ¼°ĀČ»ÆĀĮµČ£¬¼ÓČėŹŌ¼ĮĒāŃõ»ÆøĘČÜŅŗµ÷½ŚČÜŅŗµÄpHĪŖ8.0”«8.5£¬ŅŌ³żČ„ČÜŅŗÖŠÉŁĮæµÄAl3+”¢Fe3+£¬Č»ŗóŌŁ¼ÓŃĪĖįĖį»Æ£¬ŌŚĖįŠŌĢõ¼žĻĀÕō·¢½į¾§æɵƵ½CaCl2•2H2O£¬
£Ø1£©Fe3+ÓėKSCN·“Ӧɜ³ÉŗģÉ«ĪļÖŹFe£ØSCN£©3£¬¼ģŃéFe3+ŹĒ·ń“ęŌŚµÄ£¬Ń”ÓĆKSCNČÜŅŗ£»
£Ø2£©ŅņŌŚ³żŌÓ¹ż³ĢÖŠ¼ÓČėĮĖCa£ØOH£©2£¬¹Ź¼ÓČėŃĪĖįÓėČÜŅŗÖŠµÄÉŁĮæCa£ØOH£©2·“Ó¦Ź¹Ęä×Ŗ»ÆĪŖCaCl2£»ĮķCa£ØOH£©2Ņ×ĪüŹÕæÕĘųÖŠµÄCO2£¬Éś³ÉCaCO3³Įµķ£¬¹Ź¼ÓČėŃĪĖį»¹æÉŅŌ·ĄÖ¹ČÜŅŗĪüŹÕæÕĘųÖŠCO2£¬Ca£ØOH£©2ĪüŹÕæÕĘųÖŠµÄCO2£¬Éś³ÉCaCO3³Įµķ£¬Čē¹ūĪüŹÕ“óĮæµÄCO2 »įµ¼ÖĀ×īÖÕÖŹĮæ·ÖŹżĘ«µĶ£»
£Ø3£©¢ŁµĪ¶ØŹµŃéÖŠµĪ¶Ø¹ÜŠčŅŖÓĆ“ż×°ŅŗČóĻ“£¬×¶ŠĪĘæ²»ŠčŅŖČóĻ“£»
¢Śøł¾Żµ½“ļµĪ¶ØÖÕµćÉś³ÉĀČ»ÆŅųµÄĪļÖŹµÄĮæµČÓŚĻūŗĵÄĻõĖįŅųµÄĪļÖŹµÄĮæÕāŅ»¹ŲĻµĒó³öĻūŗÄĻõĖįŅųµÄĪļÖŹµÄĮ棬Ōņn£ØAgCl£©=2n£ØCaCl2.2H2O£©£¬
¾Ż“ĖæÉŅŌĖć³öŹµ¼ŹÉĻµÄCaCl2.2H2OµÄĪļÖŹµÄĮ棬½ų¶ųĒó³öÖŹĮ森עŅāČÜŅŗĢå»żŹĒ“Ó250molÖŠČ”25ml£¬ĖłŅŌŌŚ¼ĘĖ揱ŅŖ×¢ŅāÕāŅ»µć£»
¢Ūѳʷ֊“ęŌŚÉŁĮæµÄNaCl£¬øł¾Ż n£ØAgCl£©2n£ØCaCl2.2H2O£©æÉÖŖ£¬CaCl2.2H2OµÄĪļÖŹµÄĮæŌö“ó£®Ķ¬ŃłČōCaCl2.2H2OŹ§Ė®µ¼ÖĀ·ÖÄø±äŠ”£¬ÖµĘ«“ó£»
£Ø4£©½šŹōŅ±Į¶µÄ·½·ØÖ÷ŅŖÓŠ£ŗ
ČČ·Ö½ā·Ø£ŗ¶ŌÓŚ²»»īĘĆ½šŹō£¬æÉŅŌÖ±½ÓÓĆ¼ÓČČ·Ö½āµÄ·½·Ø½«½šŹō“ÓĘä»ÆŗĻĪļÖŠ»¹Ō³öĄ“£ØHg¼°ŗó±ß½šŹō£©£»
ČČ»¹Ō·Ø£ŗŌŚ½šŹō»ī¶ÆŠŌĖ³Šņ±ķÖŠ“¦ÓŚÖŠ¼äĪ»ÖĆµÄ½šŹō£¬Ķس£ŹĒÓĆ»¹Ō¼Į£ØC”¢CO”¢H2”¢»īĘĆ½šŹōµČ£©½«½šŹō“ÓĘä»ÆŗĻĪļÖŠ»¹Ō³öĄ“£ØZn”«Cu£©£»
µē½ā·Ø£ŗ»īĘĆ½šŹō½ĻÄŃÓĆ»¹Ō¼Į»¹Ō£¬Ķس£²ÉÓƵē½āČŪČŚµÄ½šŹō»ÆŗĻĪļµÄ·½·ØŅ±Į¶»īĘĆ½šŹō£ØK”«Al£©£®
½ā“š ½ā£ŗ£Ø1£©Fe3+ÓėKSCN·“Ӧɜ³ÉŗģÉ«ĪļÖŹFe£ØSCN£©3£¬¼ģŃéFe3+ŹĒ·ń“ęŌŚµÄ£¬Ń”ÓĆKSCNČÜŅŗ£¬
¹Ź“š°øĪŖ£ŗȔɣĮæÉĻ²ćĒåŅŗ£¬µĪ¼ÓKSCNČÜŅŗ£¬Čō²»³öĻÖŃŖŗģÉ«£¬Ōņ±ķĆ÷Fe£ØOH£©3 ³ĮµķĶźČ«£»
£Ø2£©Ėį»Æ²Ł×÷ŹĒ¼ÓČėŃĪĖį£¬µ÷½ŚČÜŅŗµÄpHŌ¼ĪŖ4.0£¬ĘäÄæµÄÓŠ£ŗ½«ČÜŅŗÖŠµÄÉŁĮæCa£ØOH£©2×Ŗ»ÆĪŖCaCl2£»¢Ū·ĄÖ¹ČÜŅŗĪüŹÕæÕĘųÖŠCO2£¬
¹Ź“š°øĪŖ£ŗ½«ČÜŅŗÖŠµÄÉŁĮæCa£ØOH£©2×Ŗ»ÆĪŖCaCl2£¬·ĄÖ¹ČÜŅŗĪüŹÕæÕĘųÖŠCO2£»
£Ø3£©¢ŁµĪ¶ØŹµŃéÖŠµĪ¶Ø¹ÜŠčŅŖÓĆ“ż×°ŅŗČóĻ“£¬×¶ŠĪĘæ²»ŠčŅŖČóĻ“£¬ČōČóĻ“»įŌö“ó“ż²āČÜŅŗÖŠČÜÖŹĮ棬µ¼ÖĀĻū»Æ²½ÖčČÜŅŗĢå»żŌö“󣬲ā¶Ø“ż²āŅŗµÄÅضČĘ«øߣ¬
¹Ź“š°øĪŖ£ŗ׶ŠĪĘæ£»Ę«øߣ»
¢ŚŃłĘ·ÖŠn£ØCl-£©=0.05000mol•L-1”Į0.02039L”Į10=0.010195mol£¬øł¾Żn£ØAgCl£©=2n£ØCaCl2.2H2O£©£¬Ōņn£ØCaCl2.2H2O£©=0.0050975mol£¬ĖłŅŌm£ØCaCl2.2H2O£©=0.0050975mol”Į147g/mol=0.7493325g£¬ŌņÓŠ£ŗ$\frac{0.7493225g}{0.7500g}$”Į100%=99.9%£¬
¹Ź“š°øĪŖ£ŗ99.9%£»
¢Ūѳʷ֊“ęŌŚÉŁĮæµÄNaCl»įµ¼ÖĀCaCl2.2H2OµÄĪļÖŹµÄĮæŌö“ó£®Ķ¬ŃłČōCaCl2.2H2OŹ§Ė®µ¼ÖĀ·ÖÄø±äŠ”£¬ÖµĘ«“ó£®
¹Ź“š°øĪŖ£ŗѳʷ֊“ęŌŚÉŁĮæµÄNaCl£»ÉŁĮæµÄCaCl2.2H2OŹ§Ė®£»
£Ø4£©¹¤ŅµÉĻŅ±Į¶½šŹōøʵķ½·ØŹĒµē½āČŪČŚĀČ»ÆøʵƵ½£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCaCl2 $\frac{\underline{\;Ķصē\;}}{\;}$Ca+Cl2”ü£¬
¹Ź“š°øĪŖ£ŗµē½ā·Ø£» CaCl2 $\frac{\underline{\;Ķصē\;}}{\;}$Ca+Cl2”ü£®
µćĘĄ ±¾Ģāæ¼²é»ģŗĻĪļÖŠŗ¬ĮæµÄ²ā¶Ø£¬Éę¼°ŹµŃéµÄ»ł±¾²Ł×÷”¢ŹµŃéŅĒĘ÷µÄŃ”Ōń”¢ŹµŃéĪó²ī·ÖĪö”¢»ģŗĻĪļ·ÖĄė¼°ŗ¬Įæ²ā¶ØµÄ¼ĘĖćµČ£¬×¢ŅāĄė×ӵļģŃé·½·ØŗĶ³£¼ūŅĒĘ÷µÄŹ¹ÓĆ£¬ŃłĘ·“æ¶ČµÄ·ÖĪöŅŖ×¢ŅāČÜŅŗÖŠæÉÄÜ·¢ÉśµÄ·“Ó¦£¬×¢ŅāÓŠŠ§Źż×ÖĪŹĢā£¬ĢāÄæ×ŪŗĻŠŌ½ĻĒ棬ÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģ¼ĖįµÄµēĄė·½³ĢŹ½£ŗH2CO3=2H++CO32- | |
| B£® | F-µÄ½į¹¹Ź¾ŅāĶ¼£ŗ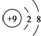 | |
| C£® | ${\;}_{55}^{134}$CsŗĶ${\;}_{55}^{137}$CsŠĪ³ÉµÄµ„ÖŹĪļĄķŠŌÖŹĻąĶ¬ | |
| D£® | NH3 µÄµē×ÓŹ½£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCl | B£® | CH3COONa | C£® | FeCl3 | D£® | NaCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ø÷ĪļÖŹµÄÅضČÖ®±Čc£ØA£©£ŗc£ØB£©£ŗc£ØC£©=2£ŗ3£ŗ4 | |
| B£® | Ę½ŗā»ģŗĻĘųĢåÖŠø÷ĪļÖŹµÄÅضČĻąµČ | |
| C£® | Ę½ŗā»ģŗĻĘųµÄĢå»żŹĒ·“Ó¦æŖŹ¼Ē°µÄ$\frac{4}{5}$ | |
| D£® | µ„Ī»Ź±¼äÄŚ£¬ČōĻūŗÄĮĖa mol AĪļÖŹ£¬Ķ¬Ź±Ņ²ĻūŗÄĮĖ2a mol CĪļÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł | B£® | ¢Ś | C£® | ¢Ū | D£® | ¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | FeS | B£® | BaSO3 | C£® | BaSO4 | D£® | S |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ü£¾¢Ū£¾¢Ł£¾¢Ś | B£® | ¢Ü£¾¢Ū£¾¢Ś£¾¢Ł | C£® | ¢Ś£¾¢Ū£¾¢Ü£¾¢Ł | D£® | ¢Ś£¾¢Ü£¾¢Ū£¾¢Ł |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com