
| ��һ�εζ� | �ڶ��εζ� | �����εζ� | |
| ������Һ�����mL�� | 25.00 | 25.00 | 25.00 |
| ����Һ�����mL�� | 9.99 | 10.01 | 10.00 |
���� ��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ��
��3������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊdz�Ϻ�ɫ��
��4���������ѧ����ʽ�����ݣ��Ӷ�����X��ֵ��
��5����6����c�����⣩=$\frac{c������V������}{V�����⣩}$���������������V�������ı仯������
��� �⣺��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧװ����ʽ�ζ����У�
�ʴ�Ϊ����ʽ��
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ�����Բ���Ҫ�������Тܢޣ�
�ʴ�Ϊ���ܢޣ�
��3�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����ɫ30s�ڲ���ɫ��˵���ζ����յ㣬
�ʴ�Ϊ���������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����30s�ڲ���ɫ��
��4�����εζ�����Ч��KMnO4����Һ��ƽ�����Ϊ10.00mL����25.00mL���������Һ���ĵ�KMnO4��
���ʵ���Ϊ0.1000mol•L-1��0.01L��100.00mL���������Һ���ĵ�KMnO4�����ʵ���Ϊ0.1000mol•L-1��0.01L��4����1.440g�����ᾧ�����ĵ�KMnO4�����ʵ���Ϊ0.1000mol•L-1��0.01L��4����2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��֪H2C2O4�����ʵ���Ϊ��0.1000mol•L-1��0.01L��4��$\frac{5}{2}$=0.01mol��0.01molH2C2O4������Ϊ0.01mol��90g/mol=0.9g������1.440gH2C2O4•xH2O��ˮ�����ʵ���Ϊ1.440g-0.9g=0.54g�������ʵ���=$\frac{0.54g}{18g/mol}$=0.03mol����x=3��
�ʴ�Ϊ��3��
��5���ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ������Һ�����С���������ʵ�����С��������С����ˮƫ�࣬Xֵƫ�ߣ�
��Ϊ��ƫ�ߣ�
��6��KMnO4����ҺŨ��ƫ�ͣ����ñ�Һ���ƫ������IJ������ʵ���ƫ�࣬����ƫ��ˮ������ƫС��XƫС��
��Ϊ��ƫ�ͣ�
���� ���⿼����������ԭ�ζ���ʵ����̷�����ʵ�����������ע��ζ��ܵ�ʹ�ú͵ζ����㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
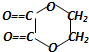 +2H2O����Ӧ������������Ӧ��D��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��HOOCCOOH+2NaHCO3=NaOOCCOONa+2CO2��+2H2O��
+2H2O����Ӧ������������Ӧ��D��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��HOOCCOOH+2NaHCO3=NaOOCCOONa+2CO2��+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
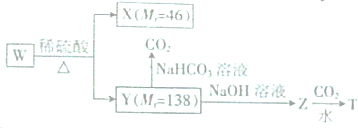
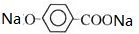 +CO2+H2O��
+CO2+H2O��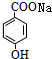 +NaHCO3��
+NaHCO3��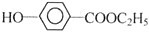
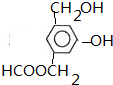 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
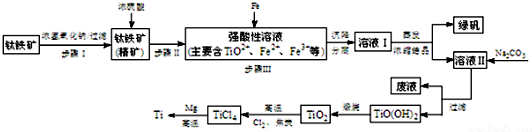
| pH | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 | Ti��OH��2 |
| ��ʼ���� | 1.1 | 4.5 | 7 | 1 |
| ��ȫ���� | 2.8 | 6.4 | 9.2 | 2.7 |
| TiCl4 | Mg | MgCl2 | Ti | |
| �۵�/�� | -25.0 | 648.8 | 714 | 1667 |
| �е�/�� | 136.4 | 1090 | 1412 | 3287 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
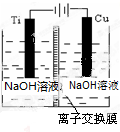 ����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����| ����a | ��̿���ڸ��������»�ԭCuO |
| ����b | �������ǻ�ԭ���Ƶ�Cu��OH��2�Ʊ�Cu2O |
| ����c | ��ⷨ����ӦΪ2Cu+H2O$\frac{\underline{\;���\;}}{\;}$Cu2O+H2�� |
| ����d | ���£�N2H4����ԭ���Ƶ�Cu��OH��2 |
| ��� | 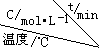 | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�� ��� | ���� ����/g | ���� ״̬ | c��H2SO4�� /mol•L-1 | V��H2SO4��/mL | ��Һ�¶�/�� | ������ʧ��ʱ��/S | |
| ��Ӧǰ | ��Ӧ�� | ||||||
| 1 | 0.10 | ˿ | 0.5 | 50 | 20 | 34 | 500 |
| 2 | 0.10 | ��ĩ | 0.5 | 50 | 20 | 35 | 50 |
| 3 | 0.10 | ˿ | 0.7 | 50 | 20 | 36 | 250 |
| 4 | 0.10 | ˿ | 0.8 | 50 | 20 | 35 | 200 |
| 5 | 0.10 | ��ĩ | 0.8 | 50 | 20 | 36 | 25 |
| 6 | 0.10 | ˿ | 1.0 | 50 | 20 | 35 | 125 |
| 7 | 0.10 | ˿ | 1.0 | 50 | 35 | 50 | 50 |
| 8 | 0.10 | ˿ | 1.1 | 50 | 20 | 34 | 100 |
| 9 | 0.10 | ˿ | 1.1 | 50 | 20 | 44 | 40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
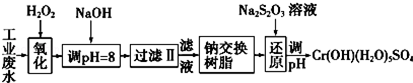
| �������� | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 | Al��OH��3 | Cr��OH��3 |
| pH | 3.7 | 9.6 | 11.1 | 8 | 9����9�ܽ⣩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com