

| 4.53g |
| 453g/mol |
| 2.16g-2.07g |
| 18g/mol |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ž��и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ѡ����
2009��4��23�����磬���ൺ��ž���ͷ��������ף�й������ž�60������ʽ�������뺣����ͧ�йص�˵���в���ȷ���� �� ��
A��Ϳ�ڽ�ͧ�ϵ���������Ϳ���еĿ�������ֱ��Լ��4mm���ң��ÿ�������ˮ��ɵĻ����ɷ������������
B����ͧ�ں�ˮ�б��ڽ����и�����ʴ������Ϊ��ˮ�к�������
C���μ��ı��Ľ�ͧ��ʹ�õ��������ǹ������ƣ���ʵ��DZͧ���ڵĿ�������
D����ͧ�ڲ�ͨ�Ų��õĹ��ˣ�SiO2���ͻ��շ��������õ��������մɶ������������ǽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
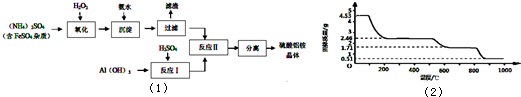
�������������մɲ��ϡ����ӹ�ҵ������ҽҩ�ȷ����й�����Ӧ��ǰ��������ͨ��������茶����ȷֽ�õ���[��֪��������茶���Ļ�ѧʽΪ(NH4)Al (SO4)n��12H2O]
�Ʊ�������茶����ʵ���������£�
 |
��1���������������С����ˡ��������Ƿ������ʵ�鷽���� ��
��2�����������У������롱�������IJ�������Ϊ�� �� �����ˡ�ϴ�ӡ����
��3���������ˮ��Һ�����Ե�ԭ���� �������ӷ���ʽ��ʾ����
��4��д����������Ӧ���л��������茶���Ļ�ѧ����ʽ ��
��5�������������Һ�м�������������Һ��Al3+�պó�����ȫ��д����Ӧ�����ӷ���ʽ ��
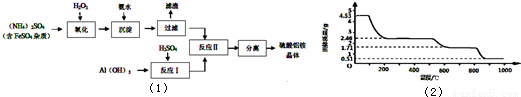
��6��ȡ4.53 g������茶�����ȷֽ⣬���ȹ����У�����������ʱ��ı仯����ͼ��ʾ��

д��400��ʱʣ�����ɷֵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�������и������ڲ��Ի�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com