
����500 mL 0.5 mol/L��NaOH��Һ,�Իش���������:
(1)����:��ҪNaOH���������Ϊ ��
(2)���Ʒ���:�������������衣
����ʢ��NaOH������ձ��м���200 mL����ˮʹ���ܽ�,����ȴ������;
�ڼ���������ƿ�м�����ˮ��Һ��ӽ��̶���1��2 cm;
�۽�NaOH��Һ�ز�����ע��500 mL����ƿ��;
��������������ˮϴ���ձ��Ͳ�����2��3��,Ȼ��ϴ��Һ��������ƿ;
�ݸ��ý�ͷ�ιܼ�����ˮ���̶���,�Ӹ�ҡ�ȡ�
�Խ�����������ȷ���� ��
(3)ijѧ��ʵ������NaOH��Һ��Ũ��Ϊ0.48 mol/L,ԭ������� ��
| A��ʹ����ֽ�����������ƹ��� |
| B������ƿ��ԭ��������������ˮ |
| C���ܽ����ձ�δ�����ϴ�� |
| D����ͷ�ιܼ�ˮ����ʱ���ӿ̶��� |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
MnO2��һ����Ҫ�������ܲ��ϣ���MnO2���ᴿ�ǹ�ҵ��������Ҫ���ڡ�ij�о���ѧϰС������˽���MnO2(���н϶��MnO��MnCO3)��Ʒת��Ϊ��MnO2��ʵ�飬���������£�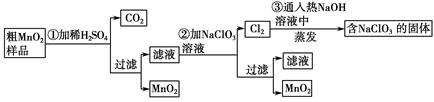
(1)�ڢٲ���ϡH2SO4ʱ����MnO2��Ʒ�е� (д��ѧʽ)ת��Ϊ���������ʡ�
(2)�ڢڲ���Ӧ�����ӷ���ʽ�� ��
�� ClO3����
ClO3���� =
= MnO2����
MnO2���� Cl2����
Cl2���� ��
��
(3)�ڢ۲������������������������̨(����Ȧ)�� �� �� ����֪�����õ��Ĺ�������NaClO3��NaOH����һ�������� (д��ѧʽ)��
(4)����MnO2��Ʒ������Ϊ12.69 g���ڢٲ���Ӧ�����˵õ�8.7 g MnO2�����ռ���0.224 L CO2(��״����)�����ڵڢڲ���Ӧ��������Ҫ mol NaClO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ȼ�����һ�ֺ���Ҫ�����Σ���Ҫ������ˮ����������Ч���á��۸���˵��ŵ㡣��ҵ�Ͽɽ���м���������У�������FeCl2����ͨ��Cl2�������Ʊ�FeCl3��Һ��
(1)����״���µ�a L�Ȼ�����������100 gˮ�У��õ���������ܶ�Ϊ
b g��mL��1�������������ʵ�����Ũ����________��
��100 mL��FeBr2��Һ��ͨ���״����Cl2 3.36 L����Ӧ�����Һ��Cl����Br�������ʵ���Ũ����ȣ���ԭFeBr2��Һ�����ʵ���Ũ��Ϊ ________��
(3)FeCl3��Һ����������ˮ���侻ˮ��ԭ��Ϊ____________________ _____________________(�����ӷ���ʽ��ʾ)����100mL 2mol��L��1��FeCl3��Һ��ˮʱ�����ɾ��о�ˮ���õ�����________0.2NA(����ڡ��������ڡ���С�ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϩ������Ļ�����干a mol����b mol O2������һ�ܱ������У���ȼ���ַ�Ӧ����ϩ������ȫ�������꣬�õ�CO��CO2�Ļ�������45 g H2O������
��1����a��1ʱ����ϩ����������ʵ���֮��n��C2H4����n��C2H6���� ��
��2����a��1���ҷ�Ӧ��CO��CO2�Ļ����������ʵ���Ϊ��ӦǰO2��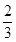 ʱ��b�� ���õ���CO��CO2�����ʵ���֮��n��CO����n��CO2���� ��
ʱ��b�� ���õ���CO��CO2�����ʵ���֮��n��CO����n��CO2���� ��
��3��a��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƷA����(NH4)2SO4��NH4HSO4�Ļ���Ϊȷ��A�и��ɷֵĺ�����ij�о���ѧϰС���ͬѧȡ��������ͬ��������ƷA����ˮ��Ȼ��ֱ���벻ͬ�����1 mol/L��NaOH��Һ��ˮԡ����������ȫ���ݳ�(���¶��£���β��ֽ�)��������������������Ũ������ȫ���ա�Ũ�������ص����������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������ͼ�ش��������⣺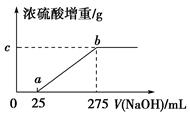
(1)д��ab���漰�����ӷ���ʽ��_____________________________��
(2)c���Ӧ����ֵ��________����ƷA��(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��.�����ʵ�����CO��CO2������Oԭ�Ӹ���֮�� ��Cԭ����֮�� �����ߵ�����֮�� ��
��2�����������У���1��Cu ��2�� Br2 ��Na2O ������ ��NaCl��Һ �� SO3 ��Ba(OH)2
���ڵ���ʵ��� �� ���ڷǵ���ʵ��� ���ܵ������ ��(�������д)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����״���£�5.6LSO2������Ϊ �ˣ������� ��ԭ�ӡ�
��2��������ͬ�� HCl��NH3��CO2��O2���������У����з�����Ŀ���ٵ��� (�����ʽ����ͬ)������ͬ�¶Ⱥ���ͬѹǿ�����£���������� ��
��3����������ƽ��ȡ5.0 g CuSO4��5H2O���壬����ˮ���100mL��Һ�������ʵ���Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ƣ���������Ӻ���(xNa2SO4��yH2O2��zH2O)����ɿ�ͨ������ʵ��ⶨ����ȷ��ȡ1.770 0 g��Ʒ�����Ƴ�100.00 mL��ҺA����ȷ��ȡ25.00 mL��ҺA�����������ữ��BaCl2��Һ��������ȫ�����ˡ�ϴ�ӡ����������أ��õ���ɫ����0.5825 g����ȷ��ȡ25.00 mL��ҺA��������ϡ�����ữ����0.020 00 mol��L��1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ25.00 mL��H2O2��KMnO4��Ӧ�����ӷ���ʽ���£�2MnO4����5H2O2��6H��=2Mn2����8H2O��5O2��
(1)��֪������BaSO4��Ksp��1.1��10��10����ʹ��Һ��c(SO42��)��1.0��10��6 mol��L��1��Ӧ������Һ��c(Ba2��)��________mol��L��1��
(2)�����ζ�������ϡ�����ữ��MnO4������ԭΪMnO2�������ӷ���ʽΪ______________��
(3)ͨ������ȷ����Ʒ�����(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������Ҫ0.80 mol��L��1 NaOH��Һ475 mL ��0. 40 mol��L��1����500 mL��������������Һ����������ش��������⣺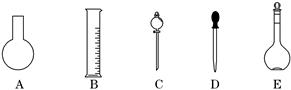
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����__________(����)������������Һ�����õ��IJ���������__________(����������)��
(2)����ƿ�����߱��Ĺ�����__________(����)��
| A������һ�����ȷŨ�ȵı���Һ |
| B����ȡһ�������Һ�� |
| C����������ƿ������µ����������Һ�� |
| D��������Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com