
�ɼ������ӻ�������ɵĻ����������������е������֣�K����NH4+��Mg2����Ba2����Cl����NO2-��SO42-��CO32-�����û��������ˮ��ó�����Һ����ȡ4��100 mL����Һ�ֱ��������ʵ�飺
| ʵ�� ��� | ʵ������ | ʵ���� |
| A | ��AgNO3��Һ | �а�ɫ�������� |
| B | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����) |
| C | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�����������Ϊ6.27 g���ڶ��γ�����������Ϊ2.33 g |
| D | ������KMnO4������Һ | KMnO4��Һ��ɫ |
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| | |
| | |
| | |
(1)����ȷ����(2)Ba2����Mg2��
(3)��NH4+��OH�� NH3����H2O
NH3����H2O
��5NO2-��2MnO4-��6H��=5NO3-��2Mn2����3H2O
(4)�����ӷ��� ���ʵ���Ũ��(mol��L��1) SO42- 0.1 CO32- 0.2 NO2- ��
(5)���ڡ�ͨ��ʵ���֪��Һ�п϶����ڵ�������NH4+��CO32-��SO42-��NO2-�������㣬NH4+�����ʵ���Ũ��Ϊ0.5 mol��L��1��CO32-��SO42-�����ʵ���Ũ�ȷֱ�Ϊ0.2 mol��L��1��0.1 mol��L��1�����ݵ���غ��K��һ������
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)����п�������ᡢ�´ɡ����¡�ҽҩ�����ӡ���ѧ�ȹ�ҵ����Ҫԭ�ϡ�����
��п��ƷΪԭ���Ʊ���������п�����������������£�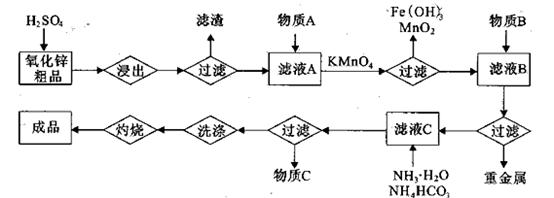
��1��.��������õ���������Һ�к���Zn2����SO42����������Fe2����Cu2����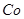 ����Mn2����
����Mn2����
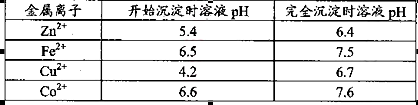
���ʡ�����A�������ǵ�����Һ��pH��5 4������A���ѡ��________��
| A��NH3.H2O | B��Na2CO3 | C��H2SO4 | D��ZnO |
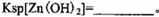 ��
��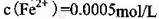 ������1
������1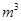 ����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���
����Һ��Fe2�������ĵ�KMnO4������Ϊ________g��������λ��Ч���֣���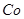 2���������û���Ӧ��ȥ��������B��_________��
2���������û���Ӧ��ȥ��������B��_________��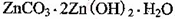 �����ɸó����Ļ�ѧ����ʽΪ________��
�����ɸó����Ļ�ѧ����ʽΪ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ������Һ��Ҫȷ���Ƿ����������ӣ�H+��K����Mg2����Al3����Fe2����Ba2����NO3����SO42����Cl����I����HCO3����ȡ����Һʵ�����£�
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ���ɫ |
| (2)ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������ˮ��ɱ��������
��1����ҵ�������缫��ʯī��Ϊ�缫��ⱥ��ʳ��ˮ�������������缫�� ����ʯī�缫�ϵĵ缫��ӦʽΪ ��
��2�������������ڼ��������£���Cl2����ˮ�е�CN-����������N2��CO2���÷�Ӧ�����ӷ���ʽΪ ��
��3���Ȱ���NH2Cl��������������Һ�ȴ�������ˮ��ͬʱͨ������������������Ӧ��Cl2 + NH3 = NH2Cl + HCl�����ɵ�NH2Cl��HClO�ȶ������ܲ���ˮ����������HClO��������ɱ�������á�
���Ȱ�������ɱ����ԭ���� ���û�ѧ�����ʾ����
���Ȱ��������������ˮ�У���Ԫ�ض���NH4+����ʽ���ڡ�
��֪��NH4+(aq) + 1��5O2(g)= NO2-(aq) + 2H+(aq) + H2O (l) ��H=��273 kJ��mol-1
NH4+(aq) + 2O2(g)= NO3-(aq) + 2H+(aq) + H2O (l) ��H =��346 kJ��mol-1
NO2-(aq)��O2������NO3-(aq)���Ȼ�ѧ����ʽΪ ��
��4����ˮ����ֳ�У�������Na2S2O3��ˮ�в������Cl2��ȥ��ijʵ��С��������ͼ��ʾװ�ú�ҩƷ�Ʊ�Na2S2O3��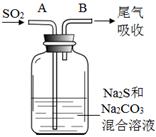

����������ϻش�
��ʼͨSO2ʱ����B�ڼ����µ��������ɣ��жϴ�B���ų����������Ƿ���H2S����д���ж����� ��
Ϊ��ý϶��Na2S2O3������Һ��pH�ӽ�7ʱ��Ӧ����ֹͣͨ��SO2����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��G�������ʼ������ͼ��ʾ��ת����ϵ������A��B��D��G����ͬ��Ԫ�ء�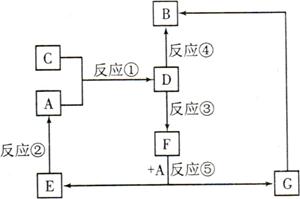
��֪��
AΪ�������ʣ�BΪ���ɫ���壬EΪ�ܶ���С�����壬GΪdz��ɫ����Һ��
D��ˮ��ҺΪ��ɫ��Һ��������������Һ��Ӧ���ɲ�����ϡ����İ�ɫ������
��ˮ��Һ��D�ܽ�ij����������ΪF��F�Ǻ�������Ԫ�صĻ����
��ش��������⣺
��1������C���ʵ�Ԫ�������ڱ��е�λ���� ���ڶ���������Ԫ���У���Ԫ����������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)��
��2��D��ˮ��Һ�� �ԣ��������ӷ���ʽ����ԭ�� ��
��3��������Ӧ�������û���Ӧ���� (�����)��
��4����Ӧ��(��D��ij������������ΪF)�����ӷ���ʽ�� ��
��5��������C��������ʵ�顣��֪������Ӧ�����У�ÿ����0.1mol KI��ת�Ƶĵ�����ԼΪ3.612��1023�����밴��Ҫ����գ�
| ʵ�鲽�� | ʵ������ | д���ӷ���ʽ |
| ����������ͨ�����KI��Һ | ��Һ������ ɫ | |
| ����ͨ������ | ��Һ�����ɫ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��W��R���ֶ�����Ԫ��ԭ���������������������X��Z��Q����Ԫ����ɣ�������0.1 mol/L����Һ��pH��13����ҵ�ϳ��õ�ⱥ��QR��Һ���ɼף�����������X��R����Ԫ����ɡ���ش��������⣺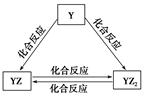
��1��Q��ԭ�ӽṹʾ��ͼΪ________��
��2��YԪ�صĵ����ܷ�����ͼ��ʾ��ת������YԪ��Ϊ________����Ԫ�ط��ţ���
�ڼ���Һ��ͨ������YZ2���壬������Һ�ʼ��ԣ�ԭ����__________________�������ӷ���ʽ�ͱ�Ҫ������˵������
��3��W�ĵ��ʼ��������Һ��Ӧ������������Һ��Ӧ��
�ٳ����£���W�ĵ��ʺͼ���Һ��ϣ�������Ӧ�����ӷ���ʽΪ______________________________________________________________________________��
��Q��W����Ԫ�ؽ����Ե�ǿ��ΪQ________W���<����>���������б�������֤����һ��ʵ����________������ţ���
a��Q���ʵ��۵��W���ʵĵ�
b��Q������������ˮ����ļ��Ա�W������������ˮ����ļ���ǿ
c��W��ԭ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ________________________�����ӷ���ʽ��_______________________��
�۶ԱȢٺ͢�ʵ�����õĽ������I2��ClO����SO42������������ǿ������˳������Ϊ________________________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��___________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________________________��
�����Ļ�ԭ�Ա�ͭǿ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�к���Fe2����Al3����Ag����Cu2����Ϊ�ֱ�õ�����һ�ֽ��������ӵij�����ɲ�ȡ��ͨ��H2S���壬��ͨ��CO2���壬�ۼ������ᣬ�ܼ�������������Һ�ĸ����裬ÿ��ͨ�������Լ�����������ÿ�ζ��ѳ������˳������������ȷ˳����(����)��
| A���ۢ٢ܢ� | B���٢ۢܢ� | C���ܢڢ٢� | D���ܢڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com