
·ÖĪö £Ø1£©ÉĻŹöĪļÖŹÖŠAl”¢NaHCO3ČÜŅŗÓėĒæĖį”¢Ēæ¼ī·“Ó¦£»
£Ø2£©µē½āÖŹ±ŲŠėĪŖ»ÆŗĻĪļ£¬ĒŅČŪČŚ»ņČÜÓŚĖ®µ¼µē£»
£Ø3£©¢ŁŗĶ¢Ž·“Ӧɜ³ÉĘ«ĀĮĖįÄĘ”¢ĒāĘų£»
£Ø4£©½įŗĻn=$\frac{m}{M}$”¢c=$\frac{n}{V}$¼ĘĖć£»
£Ø5£©A1+4HNO3=A1£ØNO3£©3+NO”ü+2H2OÖŠ£¬AlŹ§Č„µē×Ó£¬NµĆµ½µē×Ó£¬ĒŅ4molĻõĖį·“Ó¦Ź±Ö»ÓŠ1mol×÷Ńõ»Æ¼Į£»
£Ø6£©³żČ„¢ŚÖŠµÄNa2CO3£¬ĶØČė×ćĮæµÄ¶žŃõ»ÆĢ¼ĘųĢ壬·“Ӧɜ³ÉĢ¼ĖįĒāÄĘ£®
½ā“š ½ā£ŗ£Ø1£©¼ČÄÜÓėĒæĖį·“Ó¦£¬ÓÖÄÜÓėĒæ¼ī·“Ó¦µÄŹĒ¢Ł¢Ś£¬¹Ź“š°øĪŖ£ŗ¢Ł¢Ś£»
£Ø2£©µē½āÖŹ±ŲŠėĪŖ»ÆŗĻĪļ£¬ĒŅČŪČŚ»ņČÜÓŚĖ®µ¼µē£¬ŌņŹōÓŚµē½āÖŹµÄŹĒ¢Ü¢Ż¢ß£¬¹Ź“š°øĪŖ£ŗ¢Ü¢Ż¢ß£»
£Ø3£©¢ŁŗĶ¢Ž·“Ӧɜ³ÉĘ«ĀĮĖįÄĘ”¢ĒāĘų£¬Ąė×Ó·“Ó¦ĪŖ2Al+2OH-+2H2O=2AlO2-+3H2”ü£¬¹Ź“š°øĪŖ£ŗ2Al+2OH-+2H2O=2AlO2-+3H2”ü£»
£Ø4£©34.2g¢ßČÜÓŚĖ®Åä³É500mLČÜŅŗ£¬ČÜŅŗÖŠSO42-µÄĪļÖŹµÄĮæÅضČĪŖ$\frac{\frac{34.2g}{342g/mol}}{0.5L}$”Į3=0.6mol/L£¬¹Ź“š°øĪŖ£ŗ0.6mol/L£»
£Ø5£©A1+4HNO3=A1£ØNO3£©3+NO”ü+2H2OÖŠ£¬AlŹ§Č„µē×Ó£¬NµĆµ½µē×Ó£¬ĒŅ4molĻõĖį·“Ó¦Ź±Ö»ÓŠ1mol×÷Ńõ»Æ¼Į£¬ŌņøĆ·“Ó¦ÖŠ»¹Ō¼ĮÓėŃõ»Æ¼ĮµÄĪļÖŹµÄĮæÖ®±ČŹĒ1£ŗ1£¬¹Ź“š°øĪŖ£ŗ1£ŗ1£»
£Ø6£©³żČ„¢ŚÖŠµÄNa2CO3£¬ĶØČė×ćĮæµÄ¶žŃõ»ÆĢ¼ĘųĢ壬·“Ӧɜ³ÉĢ¼ĖįĒāÄĘ£¬·¢Éś·“Ó¦ĪŖNa2CO3+H2O+CO2ØT2NaHCO3£¬
¹Ź“š°øĪŖ£ŗNa2CO3+H2O+CO2ØT2NaHCO3£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄŠŌÖŹ¼°Ó¦ÓĆ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĖį¼īŃĪÖ®¼äµÄ·“Ó¦”¢µē½āÖŹµÄÅŠ¶Ļ”¢ĪļÖŹµÄĮæ¼ĘĖć¼°Ńõ»Æ»¹Ō·“Ó¦µČĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·ÖĪöÓėÓ¦ÓĆÄÜĮ¦µÄ漲飬עŅāŌŖĖŲ»ÆŗĻĪļÖŖŹ¶µÄÓ¦ÓĆ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
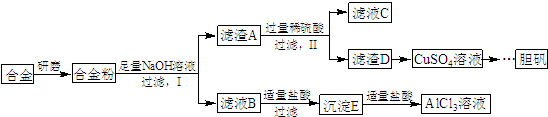
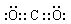 £¬Š“³öøüøÄŹŌ¼Į£ØĘųĢå¹żĮ棩ŗóÉś³É³ĮµķEĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗAlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£®
£¬Š“³öøüøÄŹŌ¼Į£ØĘųĢå¹żĮ棩ŗóÉś³É³ĮµķEĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗAlO2-+CO2+2H2O=Al£ØOH£©3”ż+HCO3-£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹żĮæĢś·ŪČÜÓŚĻ”HNO3ÖŠ£ŗFe+NO3-+4H+ØTFe3++NO”ü+2H2O | |
| B£® | ¹żĮæNaHSO4ČÜŅŗ¼ÓČėµ½Ba£ØOH£©2ČÜŅŗÖŠ£ŗ2H++SO42-+Ba2++2 OH-ØT2H2O+BaSO4”ż | |
| C£® | ÉŁĮæCl2ĶØČĖFeBr2ČÜŅŗÖŠ£ŗ2 Br-+Cl2ØT2 Cl-+Br2 | |
| D£® | ÉŁĮæ AlCl3ČÜŅŗµĪČėµ½°±Ė®ÖŠ£ŗAl3++4NH3•H2OØTAlO2-+4NH4++2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 £Ø²»æ¼ĀĒĮ¢ĢåŅģ¹¹£©
£Ø²»æ¼ĀĒĮ¢ĢåŅģ¹¹£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
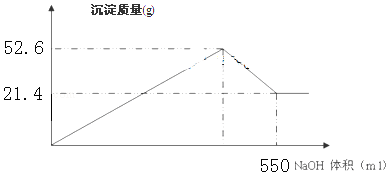
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
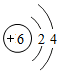 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com