
��������ˮ�����г�����ɱ��������HClO��ɱ��������ClO��ǿ��25 ��ʱ������ˮ��ϵ�д�������ƽ���ϵ��
Cl2(g)  Cl2(aq)��K1��10��1.2
Cl2(aq)��K1��10��1.2
Cl2(aq)��H2O HClO��H����Cl����K2��10��3.4
HClO��H����Cl����K2��10��3.4
HClO H����ClO����Ka����
H����ClO����Ka����
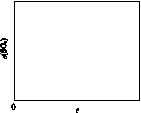
����Cl2(aq)��HClO��ClO���ֱ���������ռ����(��)��pH�仯�Ĺ�ϵ��ͼ��ʾ �����б�����ȷ����(����)
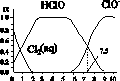
A��Cl2(g)��H2O 2H����ClO����Cl����K��10��10.9
2H����ClO����Cl����K��10��10.9
B�����ȴ���ˮ��ϵ�У�c(HClO)��c(ClO��)��c(H��)��c(OH��)
C�����ȴ�������ˮʱ��pH��7.5ʱɱ��Ч����pH��6.5ʱ��
D���ȴ�������ˮʱ�����ļ���ɱ��Ч�����ڶ�����
C��[����] ��������Ӧ����ʽ֪��K2��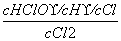 ��Ka��
��Ka��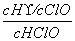 ��K��
��K�� ����K��K2��Ka������Kaδ֪������֪��K��ֵ��A��������ȴ���ˮ��ϵ�У��ɵ���غ��֪��c(H��)��c(OH��)��c(Cl��)��c(ClO��)����Cl2(aq)�� H2O
����K��K2��Ka������Kaδ֪������֪��K��ֵ��A��������ȴ���ˮ��ϵ�У��ɵ���غ��֪��c(H��)��c(OH��)��c(Cl��)��c(ClO��)����Cl2(aq)�� H2O HClO �� H�� ��Cl����HClO
HClO �� H�� ��Cl����HClO H����ClO�� ��֪c(HClO)< c(Cl��)����c(H��)>c(OH��)��c(HClO)��c(ClO�� )����c(HClO)��c(ClO��)<c(H��)��c(OH��)��B�������ͼ���е���Ϣ֪��pH��7.5����ϵ��HClO��ռ�ķ�����pH��6.5����ϵ��HClO��ռ�ķ���С������HClO��ɱ��������ClO��ǿ����pH��7.5ʱɱ��Ч����pH��6.5ʱ�C����ȷ��HClO�ĵ��������ȹ��̣����������и����HClO����ΪClO����H�������ȴ�������ˮʱ�����ļ���ɱ��Ч�����ڶ����D�����
H����ClO�� ��֪c(HClO)< c(Cl��)����c(H��)>c(OH��)��c(HClO)��c(ClO�� )����c(HClO)��c(ClO��)<c(H��)��c(OH��)��B�������ͼ���е���Ϣ֪��pH��7.5����ϵ��HClO��ռ�ķ�����pH��6.5����ϵ��HClO��ռ�ķ���С������HClO��ɱ��������ClO��ǿ����pH��7.5ʱɱ��Ч����pH��6.5ʱ�C����ȷ��HClO�ĵ��������ȹ��̣����������и����HClO����ΪClO����H�������ȴ�������ˮʱ�����ļ���ɱ��Ч�����ڶ����D�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������ұ�(CB2760��2011)�涨���Ѿ���SO2���ʹ����Ϊ0.25 g��L��1��ij��ȤС����ͼ(a)װ��(�г�װ����)�ռ�ij���Ѿ��е�SO2�������京�����вⶨ��

(a)
 ��
��
(b)
(1)����A��������__________________��ˮͨ��A�Ľ���Ϊ________��
(2)B�м���300.00 mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C�е�H2O2��ȫ��Ӧ���仯ѧ����ʽΪ________________________��
(3)��ȥC�й�����H2O2��Ȼ����0.090 0 mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ��ͼ(b)�е�________�����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ________������50 mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������(�����)________(�٣�10 mL���ڣ�40 mL����<10 mL����>40 mL)��
(4)�ζ����յ�ʱ������NaOH��Һ25.00 mL�������Ѿ���SO2����Ϊ________ g��L��1��
(5)�òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ºʹ���ѹǿ�£�����ͼ��ʾ��װ�ý���ʵ�飬���a g��CaC290%����Ʒ��ˮ��ȫ��Ӧ�������������Ϊb L����������ͬ�����¡����ij��ʯ������CaC2��������������ش��������⣺
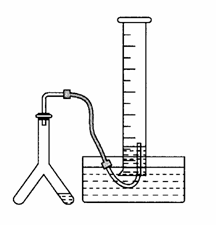
(1)CaC2��ˮ��Ӧ�Ļ�ѧ����ʽ��_______________________��
(2)����Ӧ�ս���ʱ���۲쵽��ʵ��������ͼ��ʾ����ʱ��������ȡ�������ܣ�������_________________________________________��
(3)��ʵ���в����������ʱӦע���������________________��
(4)�����ʯ��������Ϊc g������������Ϊd L����������CaC2�����������ļ���ʽ��w(CaC2)��(���������ɵ�����������Բ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)  2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
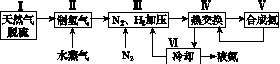
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
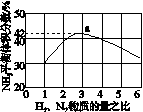 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ػ�ѧ֪ʶ�����жϣ����н��۴������(����)
A��ij���ȷ�Ӧ���Է����У���˸÷�Ӧ��������Ӧ
B��NH4Fˮ��Һ�к���HF�����NH4F��Һ���ܴ���ڲ����Լ�ƿ��
C����ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں���
D������Ӧ��Ũ�ȿɼӿ췴Ӧ���ʣ������Ũ����������Ӧ����������H2������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£�N2O�ֽ�IJ���ʵ���������£�
| ��Ӧʱ�� /min | 0 | 10 | 20 | 30 | 40 | 50 |
| c(N2O) (mol��L��1) | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 |
| ��Ӧʱ�� /min | 60 | 70 | 80 | 90 | 100 | |
| c(N2O) (mol��L��1) | 0.040 | 0.030 | 0.020 | 0.010 | 0.000 |
��ͼ����ȷ��ʾ�÷�Ӧ�й��������仯���ɵ���(����)
(ע��ͼ�а�˥��ָ��һŨ��N2O����һ��ʱ�������Ӧʱ�䣬c1��c2����ʾN2O��ʼŨ����c1<c2)
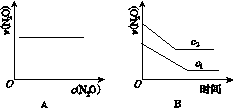
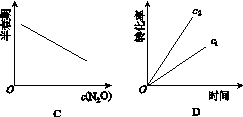
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ú̿ȼ�չ����л��ͷų�������SO2�������ƻ���̬����������һ����������������Ԫ����CaSO4����ʽ�̶����Ӷ�����SO2���ŷš�����ú̿ȼ�չ����в�����CO�ֻ���CaSO4������ѧ��Ӧ����������Ч�ʡ���ط�Ӧ���Ȼ�ѧ����ʽ���£�
CaSO4(s)��CO(g) CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaO(s) �� SO2(g) �� CO2(g)����H1��218.4 kJ��mol��1(��Ӧ��)
CaSO4(s)��4CO(g) CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
CaS(s) �� 4CO2(g)����H2����175.6 kJ��mol��1(��Ӧ��)
��ش��������⣺
(1)��Ӧ���ܹ��Է����еķ�Ӧ������________��
(2)�����������ķ�Ӧ����ʾƽ�ⳣ��Kpʱ���������(B)��ƽ��ѹǿp(B)������������ʵ�����Ũ��c(B)����Ӧ���Kp��________(�ñ���ʽ��ʾ)��
(3)����ij�¶��£���Ӧ�������(v1 )���ڷ�Ӧ�������(v2 )�������з�Ӧ���������仯ʾ��ͼ��ȷ����________��
(4)ͨ����ⷴӦ��ϵ������Ũ�ȵı仯���жϷ�Ӧ��͢��Ƿ�ͬʱ������������____________________________________________________________________��
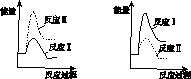
�������������� ��A������������B
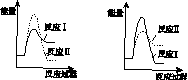
�������������� ��C������������D
(5)ͼ(a)Ϊʵ���ò�ͬ�¶��·�Ӧ��ϵ��CO��ʼ����ٷ�����ƽ��ʱ���������CaS�����ٷ����Ĺ�ϵ���ߡ��÷�Ӧ��ϵ��SO2�������Ĵ�ʩ��________��
A����÷�Ӧ��ϵ��Ͷ��ʯ��ʯ
B���ں��ʵ��¶�������ƽϵ͵ķ�Ӧ�¶�
C�����CO�ij�ʼ����ٷ���
D����߷�Ӧ��ϵ���¶�
(6)���º��������£����跴Ӧ��͢�ͬʱ��������v1>v2������ͼ(b)������Ӧ��ϵ��c(SO2)��ʱ��t�仯��������ͼ��
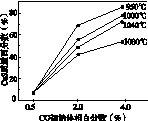 ��
��
����������(a)������������������������(b)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ƽ��״̬�ķ�Ӧ2H2S(g)  2H2(g)��S2(g)����H>0�����ı���������������º�����˵����(����)
2H2(g)��S2(g)����H>0�����ı���������������º�����˵����(����)
A�������������Ӧ·���������ı䣬��HҲ����֮�ı�
B�������¶ȣ����淴Ӧ���ʶ�����H2S�ֽ���Ҳ����
C������ѹǿ��ƽ�����淴Ӧ�����ƶ�����������ϵ�¶Ƚ���
D������ϵ���ݣ�ע��һЩH2�����ƽ�⣬H2Ũ�Ƚ���С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ����һ�����������Σ�Ϊ��ˮ��ϵ�����л������ӡ�Al2Cl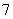 ��AlCl
��AlCl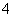 ��ɵ�����Һ�������Һʱ�����ڸ���Ʒ�ϵ������
��ɵ�����Һ�������Һʱ�����ڸ���Ʒ�ϵ������
(1)����Ʒ�ӵ�Դ��________������֪��ƹ����в����������������л������Ӳ�����缫��Ӧ�������缫��ӦʽΪ__________________________________________��������AlCl3ˮ��Һ�����Һ������������Ϊ________��
(2)Ϊ�ⶨ�Ʋ��ȣ���NaOH��Һ�ܽ����Ʒ��������Ʋ㣬����Ӧת��6 mol����ʱ�����û�ԭ��������ʵ���Ϊ________mol��
(3)�����ۺ�Fe2O3�����ȷ�Ӧʵ�飬��Ҫ���Լ�����________��
a��KCl b��KClO3 c��MnO2 d��Mg
ȡ�������ȷ�Ӧ���õĹ������������������ϡH2SO4���μ�KSCN��Һ����������______________(��ܡ��� ���ܡ�)˵��������������Fe2O3��������________(�����ӷ���ʽ˵��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com