
��������(SnSO4)��һ����Ҫ��������ˮ�������Σ��㷺Ӧ���ڶ�����ҵ��ij�о�С��
���SnSO4�Ʊ�·�����£�
�������ϣ�
I�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ�������
��SnC12��ˮ�����ɼ�ʽ�Ȼ�����[Sn(OH)Cl]
�ش��������⣺
��1������l�IJ���Ϊ ____��____�����ˡ�ϴ�ӡ�����Գ�������ϴ�ӵķ�����_____________��
��2�� SnCl2��ĩ���Ũ��������ܽ⣬���ϱ�Ҫ�Ļ�ѧ����ʽ��ƽ���ƶ�ԭ������ԭ��________��
��3������Sn�۵��������������ٵ�����ҺpH;��__________��
��4�����������£�SnSO4����������˫��ˮȥ������������Ӧ�����ӷ���ʽ�ǣ�________________��
��5����С��ͨ�����з����ⶨ�������۵Ĵ��ȣ����ʲ����뷴Ӧ����ȡag�������������У������ɵ�SnC12�м��������FeC13��Һ����b mol/LK2Cr2O7�ζ����ɵ�Fe2+����֪���Ի����£�Cr2O72-�ɱ���ԭΪCr3+��������ȥK2Cr2O7��Һm ml������������������������________����Sn��Ħ������ΪM g/mol���ú�a��b��m��M�Ĵ���ʽ��ʾ��
��1������Ũ������ȴ�ᾧ������������©���У�������ˮ��û������ʹˮ��Ȼ���£��ظ�����2-3��
��2��SnCl2 + H2O Sn(OH)Cl + HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ��
Sn(OH)Cl + HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ��
��3����ֹSn2+������
��4��Sn2+ + H2O2 +2H+ = Sn4+ + 2H2O
��5��3bmM /1000a
���������������1������SnSO4���ܽ�����¶ȵ�Ӱ��仯�ϴ����¶ȵ����߶��������¶ȵĽ��Ͷ���С�����Դ�SnSO4��Һ�л��SnSO4����ķ���������Ũ������ȴ�ᾧ��ϴ�ӳ����ķ����ǽ���������©���У�������ˮ��û������ʹˮ��Ȼ���£��ظ�����2-3�Ρ���2�� SnCl2��ĩ���Ũ��������ܽ⣬����ΪSnCl2��ǿ�������Σ���ˮ��������ˮ�ⷴӦSnCl2 + H2O Sn(OH)Cl + HCl������HCl���ܽ⣬�����������������Ũ�ȣ�����ʹˮ��ƽ�����淴Ӧ�����ƶ���������SnCl2��ˮ�ⷴӦ�ķ�������3�� ���������������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ����������Լ���Sn�۵��������������ٵ�����ҺpH;�ڷ�ֹSn2+����������5���йط�Ӧ�ķ���ʽΪSn+2HCl=SnCl2+H2����SnCl2+2FeC13=SnCl4+2FeC12�� 6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O���ɷ���ʽ�ù�ϵʽΪ3Sn����6 FeC12����K2Cr2O7��n(Cr2O72-)=mb��10-3mol.����n(Sn)=3mb��10-3mol.������������������������(3mb��10-3mol��Mg/mol)��ag="3bmM" /1000a.
Sn(OH)Cl + HCl������HCl���ܽ⣬�����������������Ũ�ȣ�����ʹˮ��ƽ�����淴Ӧ�����ƶ���������SnCl2��ˮ�ⷴӦ�ķ�������3�� ���������������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��Sn2+�ױ����������Լ���Sn�۵��������������ٵ�����ҺpH;�ڷ�ֹSn2+����������5���йط�Ӧ�ķ���ʽΪSn+2HCl=SnCl2+H2����SnCl2+2FeC13=SnCl4+2FeC12�� 6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O���ɷ���ʽ�ù�ϵʽΪ3Sn����6 FeC12����K2Cr2O7��n(Cr2O72-)=mb��10-3mol.����n(Sn)=3mb��10-3mol.������������������������(3mb��10-3mol��Mg/mol)��ag="3bmM" /1000a.
���㣺�������������ϴ�ӳ����ķ������ε�ˮ��ƽ���ƶ�������ʽ����д����ϵʽ���ڼ������ʴ��ȵļ����Ӧ�õ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʽ�Ȼ���[ Al2(OH)nCl6-n ] �����ù�ҵ���Һͻ���������Ϊԭ�ϣ���Ҫ��Al��Al2O3��SiO2������������������Ƽӹ����ɣ��˲�Ʒ���Խϸߣ��Թ�ҵ��ˮ���нϺõľ���Ч�������Ʊ��������£�
��1��ԭ����Ҫ���飬��Ŀ���� ������I����Ҫ�ɷ��� ��
��2�����������й����У���Һ��Ϊdz��ɫ����ʱ��Һ�г�dz��ɫ�������ӳ����ü��� �Լ����м��飨�ѧʽ���������Һ�ֱ�Ϊ�ػ�ɫ����ط�Ӧ�����ӷ���ʽΪ ��
��3��������м���������Ca(OH)2������pH����Ŀ�ģ�һ�����ɼ�ʽ�Ȼ��������� ����֪��ʽ�Ȼ����ķ�ɢ�����Ӵ�С��1~100 nm֮�䣬��������ҺI���ʽ�Ȼ�������Һ������������� ����Ca(OH)2��Һ����������۵õ��ļ�ʽ�Ȼ�������ƫ�ͣ��÷�Ӧ�����ӷ���ʽΪ ��
��4��ij�¶�����0.1 mol AlCl3��������ˮ������2.5%ˮ������Al(OH)3��Һʱ����������Q kJ ���ù��̵��Ȼ�ѧ��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�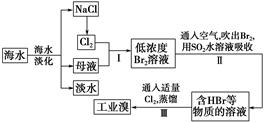
��1�����оٺ�ˮ���������ַ�����________��________��
��2����������ѻ��Br2����������ֽ�Br2��ԭΪBr������Ŀ����_________��
��3���������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ_______��
�ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������_______��
��4��ij��ѧ�о���ѧϰС��Ϊ���˽�ӹ�ҵ�����ᴿ��ķ������������й�����֪��Br2�ķе�Ϊ59 �棬����ˮ���ж�����ǿ��ʴ�ԡ����Dzι��������̺�������װ�ü�ͼ��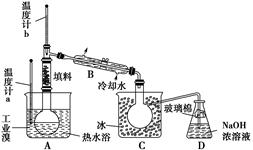
�������������ۣ�
��ͼ������B��������____________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����__________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�������___________��
��C��Һ����ɫΪ________________��Ϊ��ȥ�ò������Բ���������Cl2���������м���NaBr��Һ����ַ�Ӧ���ٽ��еķ��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���ۺ����ÿ����Ʊ�����þ������������ͼ��ʾ��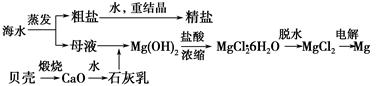
(1)Mg(OH)2�����л��е�Ca(OH)2Ӧ������ȥ��д��ʵ�鲽�衣__________________________
(2)ʵ���ҽ������Ƴɾ��εĹ����У��ܽ⡢���ˡ�������������IJ�����Ҫ�õ����������ֱ�˵���������������ʹ�ò�������Ŀ�ģ�
���ܽ⣺________________��
�ڹ��ˣ�__________________________��
��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ų�������ʯ�͡���������ˮ�������������ճ�������Ʒ����ҵ�ƹ㣬�ѽ������汻�������ӣ�����Ϊ���ִ��������͡�ս�Խ�����������߹���װ��ˮƽ���ɻ�ȱ����Ҫս�����ʡ���ҵ��Ҫ�Զ�������Ϊԭ��ұ�������ѡ�
��.�������ѿ����������ַ����Ʊ���
����1�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�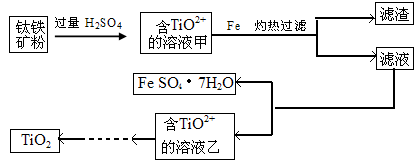
��1������Һ����̷�����IJ��������� ��
��2������Һ�г���TiO2+֮����еĽ����������� ��
��3����֪10kg������������Ԫ�ص���������Ϊ33.6%���ܹ��õ��̷�����22.24kg���Լ������ټ������۵�������
����2��TiCl4ˮ������TiO2��XH2O�����ˡ�ˮϴ��ȥ���е�Cl-���ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��4����TiCl4ˮ������TiO2��XH2O�Ļ�ѧ����ʽΪ ��
�ڼ���TiO2��XH2O��Cl-�Ƿ����ķ����� ��
��.�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�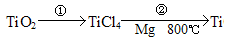
��Ӧ�ڵĻ�ѧ����ʽ�� ���÷�Ӧ�ɹ���Ҫ������������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Һ�žú���������������AgN3��������ը��ֱ���ŷŻ���Ⱦ���������������Դ���˷ѡ�ij�о�С������˴�������Ӧ��ķ�Һ�У���������������Һ�����費��������������������������ʵ�����̣�
����֪��[Ag��NH3��2]������Һ�д���ƽ�⣺[Ag��NH3��2]��??Ag����2NH3��
��1��д�������ڢٲ���Һ��ϡHNO3��Ӧ�����ӷ���ʽ ��
��2�������ڢڲ����������Ҫ������Ŀ���� ��
�������̿��ܲ����Ĵ�����Ⱦ���� ��
��3���ҷ��������յõ����۵�����ƫ���ų�δϴ�Ӹɾ������أ����ܵ�ԭ���� ��
��4��ʵ��������������Һ�IJ��������� ��
��5����֪�ҷ����ڢ۲���Ӧ��H2S��������������յõ�����21.6 g��������������ʧ�������ϸò���Ҫ�������� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�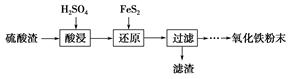
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʯ���顢ʯ�ҵ�(CaCN2)��������(��H2S)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��CS(NH2)2(����)���䲿�ֹ����������£�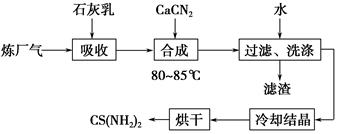
(1)�����£�H2S�������з�Ӧ��2H2S(g)??2H2(g)��S2(g)����ƽ�ⳣ������ʽΪK��________________��
(2)��ʯ��������H2S��ȡCa(HS)2��Ҫ�ڵ����½��У���ԭ����_____________________________________________________��
���˵õ��������������ã���������Ҫ�ɷ���________(�ѧʽ)��
(3)�ϳ������賤ʱ����裬���ڽϸ��¶�(80��85 ��)�½��У���Ŀ����_______________________________________��
Ca(HS)2��CaCN2��ˮ��Һ�кϳ�����Ļ�ѧ����ʽΪ________________________________��
(4)������X�����廥Ϊͬ���칹�壬X����FeCl3��Һ�У���Һ�Ժ�ɫ��X�Ļ�ѧʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ײ��϶������ѣ�TiO2�����кܸߵĻ�ѧ���ԣ��������������Ĵ�����
��1����ҵ�϶������ѵ��Ʊ������ǣ�
��.�������Ľ��ʯ����Ҫ�ɷ���TiO2����Ҫ������SiO2����̼�ۻ�Ϸ����Ȼ�¯�У��ڸ�����ͨ��Cl2��Ӧ���Ƶû���SiCl4���ʵ�TiCl4��
��.��SiCI4���룬�õ�������TiCl4��
��.��TiCl4�м�ˮ�����ȣ�ˮ��õ�����TiO2��xH2O��
��.TiO2��xH2O���·ֽ�õ�TiO2��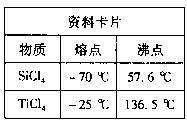
�ٸ������Ͽ�Ƭ����Ϣ�жϣ�TiCl4��SiCl4�ڳ����µ�״̬�� ��������������õIJ��������� ��
�ڢ��з�Ӧ�Ļ�ѧ����ʽ�� ��
�������ʵ��������ɣ�Ӧ��TiO2��xH2O���� ������ĸ���м��ȡ�
��2���ݱ���������̬��·����������ʱ����һ������TiO2��TiO2��̫������������ĵ��ӱ�������ˮ�е�����ã�����H2O2��H2O2�����·������е�CxHy��CO�ȣ�����Ҫ��������H2O2�� ��������ԡ���ԭ�ԡ�����
��3��ij�о�С��������װ��ģ�⡰��̬��·���IJ���ԭ�������г�װ������ȥ��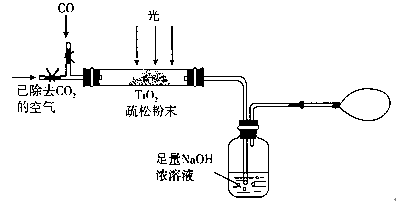
���绺��ͨ��22.4 L��������ɱ�״����CO���壬���NaOH��Һ����11 g����CO��ת����Ϊ ��
�ڵ�CO����ȫ��ͨ���Ҫ��ͼʾͨһ��ʱ���������Ŀ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com