
ij��֪A��B���������ֶ�����Ԫ����ɵĻ����A��ijԪ�ص���������Ϊ25%��B����ɫ��Ӧ�ʻ�ɫ��C��J��X��ͬ���ڵ�Ԫ�صļ��⻯�XΪ��ɫҺ�壬C��JΪ���壬D��һ�ֲ�����ˮ�İ�ɫ���塣��Ӧ���ɵ�ˮ������ȥ������������ͼ��ʾ�Ĺ�ϵ��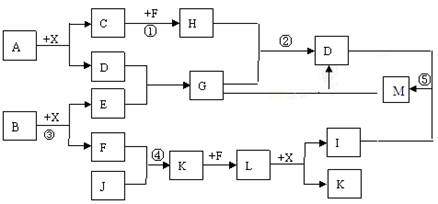
��1��д����ѧʽ��A_________ E___________ L___________��
��2���ڷ�Ӧ�٢ڢۢܢ�������������ԭ��Ӧ����_____________��
��3����Ӧ�ۻ�ѧ����ʽΪ��______________________________��
��4��д���������ӷ���ʽ����Ӧ�� ��G��Һ��M��Һ�ķ�Ӧ___________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������������õķ���ʴ�ԣ��ڹ�����ҵ���зdz���Ҫ�����ã��������ȷ�Ӧ��ɸֹ�ĺ��ӷdz�����Ѹ�١����������գ�
��1��������©���н����ȼ���Ͼ��Ⱥ��������ȷ�Ӧ�IJ����ǣ� ��
��2��������ͬ���ڡ��ؿ���衢���ĺ����� �����,����=����SiO2�ǹ����β�����Na2CaSi6O14����Ҫ�ɷ֣�Na2CaSi6O14Ҳ��д��Na2O��CaO��6SiO2���Ƴ�ʯ��NaAlSi3O8������������ʽ ����ʯ���������Σ���ͬ�ʯ����ԭ�ӵ����ʵ���������ͬ���ɴ˿���֪�Ƴ�ʯ�Ļ�ѧʽΪ ��
��3��ij���Ͻ���Al��Si��Cu��Mg��ɡ��ٳ�ȡ100g�����Ͻ���Ʒ���ֳɵ�������A��B���ݡ���A�ݼ�������NaOH��Һ��B�ݼ���������ϡ���ᡣ�ڴ����ݷ�Ӧ�ﶼ��ַ�Ӧ֮�Ƶ������������1.60g���ռ��õ������������������2240mL(��״����)������Ʒ��Si��Mg�����ʵ����ֱ��� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Li/FeS2��ص������������ʣ�����ˮ�ȷ��ϳɡ�FeSO4��Na2S2O3��S��H2O��200��������Ӧ24Сʱ�����������Ե����ʵ�����Ӧ����������CS2��H2Oϴ�ӡ����P������õ���
��1���ϳ�FeS2���ӷ���ʽΪ ��
��2����ˮϴ��ʱ�����֤��S042-�������� ��
��3����֪1.20gFeS2��O2����ȫȼ������Fe2O3��SO2����ų�8.52kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ�� ��
��4��ȡ�����Ƶõ���������1.1200g (�ٶ�ֻ��FeSһ������)�����������������г�ּ��ȣ�����0.8000g����ɫ���壬�������������FeS2����������(д���������)��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���ѧ����Cl2��FeCl2��KSCN�����Һ�ķ�Ӧ����ʵ��̽����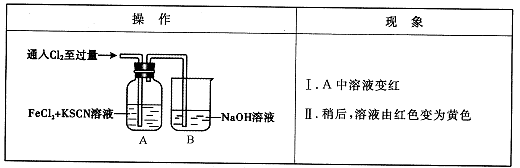
��1��B�з�Ӧ�����ӷ���ʽ��____��
��2��A����Һ����ԭ����____��
��3��Ϊ��̽������II��ԭ��ͬѧ��������ʵ�顣
��ȡA�л�ɫ��Һ���Թ��У�����NaOH��Һ���к��ɫ�������ɣ�����Һ��һ������ ��
��ȡA�л�ɫ��Һ���Թ��У����������KSCN��Һ�����յõ���ɫ��Һ��
��ͬѧ��ʵ��֤������������ԭ����SCN����Cl2�����˷�Ӧ��
��4����ͬѧ����SCN�����ܱ�Cl2�����ˣ����ֽ����������о���
������ʾ��SCN���ĵ���ʽΪ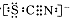
�ټ�ͬѧ��ΪSCN����̼Ԫ��û�б������������� ��
��ȡA�л�ɫ��Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN���б�������Ԫ���� ��
��ͨ��ʵ��֤����SCN���е�Ԫ��ת��ΪNO3-������ʵ�鷽����____��
����SCN����Cl2��Ӧ����1 mol CO2����ת�Ƶ��ӵ����ʵ����� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(6��) ��1L AlCl3��FeCl3�����Һ�м���2mol /LNaOH��Һ300mLʱ�������ij����������ֵ����������NaOH��Һ��������ʼ�ܽ⣬������NaOH��Һ��������ﵽ 350mLʱ���������ټ��٣���ԭ��Һ��FeCl3�����ʵ���Ũ��(��д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��12�֣���ʽ̼����þ[MgaAlb(OH)c(CO3)d��x H2O]������������ȼ����
��1����ʽ̼����þ������ȼ���ã������������ȷֽ������մ��������� ��
��2��MgaAlb(OH)c(CO3)d��x H2O��a��b��c��d�Ĵ�����ϵʽΪ ��
��3��Ϊȷ����ʽ̼����þ����ɣ���������ʵ�飺
��ȷ��ȡ3.390g��Ʒ������ϡ�����ַ�Ӧ������CO20.560L���ѻ���ɱ�״���£�������ȡһ������Ʒ�ڿ����м��ȣ���Ʒ�Ĺ�������ʣ�������Ʒ��ʣ������/������Ʒ����ʼ������100%�����¶ȵı仯����ͼ��ʾ����Ʒ��2700Cʱ����ȫʧȥ�ᾧˮ��6000C���ϲ�������Ϊ����������Ļ�����
��������ʵ�����ݼ����ʽ̼����þ��Ʒ�е�n(OH��): n(CO32��)��д��������̣���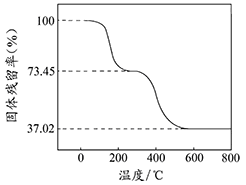
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣�����22g Mg��Al��Zn��Fe���ֻ��ý�����ĩ�Ļ������200mL����һ��������20%������Һǡ����ȫ��Ӧ���õ���ˮ��70g����Ҫ��д��������̣�
��1��������Һ��������
��2�����ɵ������������״������
��3���������ʵ���Ũ�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����þ�������ۡ�ͭƬ����������������������ؼ��������õ�ʵ����Ʒ����������������ҩƷ��������Ƽ�ʵ����֤�������������ʵĻ�ԭ��ǿ������������Ƶ�ʵ�鷽�����ش��������⣺
��1��ʵ��ԭ�������û�ѧ����ʽ��ʾ�� ��ʵ��������Ļ�ѧ����Ϊ ��
��2������ʵ��IJ�������Ϊ�� ��
��3��ʵ��̽����ȡ������Ӧ���õ��Ĺ������������������ϡH2SO4���μ�KSCN��Һ���������� �����ܻ��˵��������������Fe2O3����˵������ �����������ӷ���ʽ˵������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧ��ȤС���������ʵ�鷽�����ⶨij�Ѳ��ֱ��ʵ�С�մ���Ʒ��Na2CO3������������
����һ����ȡһ��������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣
(1)�����з�����Ӧ�Ļ�ѧ����ʽΪ��________��
(2)ʵ���У�����������ص�Ŀ����________��
����������ȡһ��������Ʒ������С�ձ��У�������ˮ�ܽ⣻��С�ձ��м�������Ba(OH)2��Һ�����ˣ�ϴ�ӡ���������������������������㡣(��֪��Ba2����OH����HCO3��=BaCO3����H2O)
(1)���˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ________��
(2)ʵ�����жϳ����Ƿ���ȫ�ķ�����________��
��������������ͼ��ʾװ�ý���ʵ�飺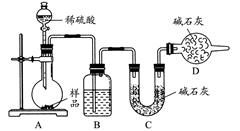
(1)Dװ�õ�������________����Һ©����________(��ܡ����ܡ�)���������ϡ�������ʵ�顣
(2)ʵ��ǰ��ȡ17.90 g��Ʒ��ʵ�����Cװ������8.80 g������Ʒ��Na2CO3����������Ϊ________��
(3)���ݴ�ʵ���õ����ݣ��ⶨ���������Ϊʵ��װ�û�����һ������ȱ�ݣ���ȱ����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com