
| ʵ �� �� �� | �� �� �� ʵ �� �� �� |
| ��1�����л�������A�������Dzⶨʱ����÷������������Ƭ����Է�������Ϊ90�� | ��ͨ��������գ� ��1��A��Ħ������Ϊ�� |
| ��2������9.0gA��������O2���ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g�� | ��2��A�ķ���ʽΪ�� |
| ��3����ȡA 9.0g����������NaHCO3��ĩ��Ӧ������2.24LCO2����״�������������������Ʒ�Ӧ������2.24LH2����״������ | ��3�����Ƴ�A�к��еĹ����ţ���д���ƣ� |
��4��A�ĺ˴Ź���������ͼ�� | ��4��A�к��� |
| ��5������������A�Ľṹ��ʽ | |
| 9.0g |
| 90g/mol |
| 2.24L |
| 22.4L/mol |
| 2.24L |
| 22.4L/mol |
| 13.2g |
| 44g/mol |
| 5.4g |
| 18g/mol |
| 9.0g-0.3mo��12g/mol-0.3mol��2��1g/mol |
| 16g/mol |
| 9.0g |
| 90g/mol |
| 2.24L |
| 22.4L/mol |
| 2.24L |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������һ����������������Ļ��������Ժ��ֱ�����ϣ���ȫȼ�յĺ��������� |
| B�������ʵ���һ�����Ҵ�����ϩ�Ļ��������Ժ������ʵ���������ϣ���ȫȼ�յĺ��������� |
| C��������һ���ĵ��ۺ���ά�ػ��������Ժ��ֱ�����ϣ���ȫˮ���ˮ������ |
| D��������һ������֬�������Ժ��ֱ�����ϣ���ȫˮ������ɸ��͵������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������� |
| B�������¶� |
| C��ʹˮ�ֽ� |
| D��ʹˮ���ˮ�����Իӷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2��4-����-1-��ϩ�� ��
��2��4-����-1-��ϩ�� ��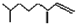 ����CH3��CH2��2CH=CH-CH=CH��CH2��8CH3���ش��������⣺��1���ķ���ʽ
����CH3��CH2��2CH=CH-CH=CH��CH2��8CH3���ش��������⣺��1���ķ���ʽ ���л���
���л��� ��M��Ϊͬ���칹�壬����̼��������Һ��Ӧ�ų�CO2���л�����X���ܵĽṹ��
��M��Ϊͬ���칹�壬����̼��������Һ��Ӧ�ų�CO2���л�����X���ܵĽṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B |
| ����ʹ������Ȼ�̼��Һ��ɫ �ڱ���ģ��Ϊ��  ������ˮ��һ�������·�Ӧ���ɴ� | ����C��H����Ԫ����� �����ģ��Ϊ�� 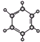 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com