
(12��)
������Ԫ����ɵĵ���X2��Y����״����X2���ܶ�Ϊ3.17g��L��1�������£�YΪdz��ɫ���塣Z��һ�ֻ������ɫ��Ӧ��dz��ɫ(���ܲ���)��0.1mol��L��1 Z��ˮ��ҺpH=13��X2��Y ��Z֮��������ת����ϵ(��������������ȥ)
 ��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��2����֪C�������ᷴӦ������ʹƷ����Һ��ɫ������
��D�Ļ�ѧʽ�� ��D��ˮ��ҺpH��7��ԭ����(�����ӷ���ʽ��ʾ)
�ڽ�20mL 0.5mol��L��1 C��Һ��μ��뵽20 mL 0.2mol��L��1 KMnO4��Һ(�����ữ)�У���Һǡ����Ϊ��ɫ��д����Ӧ�����ӷ���ʽ
���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�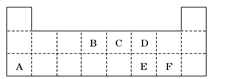
ij�ֽ���Ԫ�صĵ���G�����Է�������ͼ��ʾת����
���л�����M��һ�ְ�ɫ��״������K����Һ�����B��ij�������ﷴӦ�Ļ�ѧ����ʽΪ___________________________��һ���������ǽ���������GԪ�غ�CԪ����ɣ��仯ѧʽΪ ��
��1�� Cl2 �� 2OH�C = Cl�C �� ClO�C �� H2O
��2�� �� K2S S2�C �� H2O  HS�C �� OH�C
HS�C �� OH�C
�� 5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O
��NaAlO2 �� CO2 �� 2H2O = NaHCO3 �� Al(OH)3�� ��AlN
���������������M(X2) = 3.17g��L��1��22.4L��mol�C1 =" 71" g��mol�C1������XΪ��Ԫ�أ������£�������Ԫ��Y�ĵ���Ϊ����ɫ���壬��YΪ��Ԫ�أ�Z����ɫ��Ӧ��dz��ɫ��˵��Z�к��м�Ԫ�أ�0.1mol��L��1 Z��ˮ��ҺpH=13˵��ZΪǿ���ZΪKOH����X2��Y ��Z֮���ת����ϵͼ��֪X2��Z�ķ�ӦΪ��Cl2 + 2KOH =" KCl" + KClO + H2O��Y ��Z�ڼ��������µķ�ӦΪ��3S + 6KOH = K2S + 2K2SO3 + 3H2O�����������ɵã���1�� д�������µ���X2��Z��Ӧ�����ӷ���ʽ��Cl2 �� 2OH�C = Cl�C �� ClO�C �� H2O ��2��CΪK2SO3�������ᷴӦ����SO2ʹƷ����Һ��ɫ����DΪK2S����D�Ļ�ѧʽΪK2S����ˮ��ҺpH��7������ΪS2�Cˮ��ʼ��ԣ�ˮ������ӷ���ʽΪ��S2�C �� H2O  HS�C �� OH�C����n(K2SO3)= 0.02L��0.5mol��L�C1 = 0.01mol��n(KMnO4) = 0.02L��0.2mol��L��1 = 0.004mol��n(K2SO3):n(KMnO4) =5:2������KMnO4����ǿ�����ԣ��ܰ�����ΪSO42�C����������ԭΪMnO4�C������ƽ������ӷ���ʽΪ��5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O��
HS�C �� OH�C����n(K2SO3)= 0.02L��0.5mol��L�C1 = 0.01mol��n(KMnO4) = 0.02L��0.2mol��L��1 = 0.004mol��n(K2SO3):n(KMnO4) =5:2������KMnO4����ǿ�����ԣ��ܰ�����ΪSO42�C����������ԭΪMnO4�C������ƽ������ӷ���ʽΪ��5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O��
��������G����NaOH��Һ��Ӧ����GΪ��Ԫ�أ���Ԫ�����ڱ�������Ԫ�ص�λ�ÿ�֪��BΪ̼Ԫ�أ�CΪ��Ԫ�أ�FΪ��Ԫ�أ��ִ��й����ʵ�ת����ϵͼ��֪��KΪƫ�����ƣ�LΪ�Ȼ�����KΪ������������NaAlO2��CO2��Ӧ�Ļ�ѧ����ʽΪ��NaAlO2 �� CO2 �� 2H2O = NaHCO3 �� Al(OH)3�������뵪Ԫ���γɻ�����ʱ������+3�ۣ���Ϊ�C3�ۣ��仯ѧʽΪAlN��
���㣺������Ҫ���������ƶϣ���ͬʱ�漰��֪ʶ��϶࣬���ε�ˮ�⡢���ӷ���ʽ����д��������ԭ��Ӧ������ƽ�����ǽ������ϵ����ݣ���Ҫ����ѧ����֪ʶ������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��)��֪��������A�������������������Ľ����� D��������ˮ�İ�ɫ���塣 FΪ���ɫ���塣��ɫ�������������������ɺ���ɫ�������ҡ���ͼ�в��ֲ���ͷ�Ӧ�������ԣ���
�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽC__________G__________��
д����Ӧ�ٵĻ�ѧ����ʽ________________________________ ��
��Ӧ�ڵ����ӷ���ʽ_____________________________��
��3����Ӧ���������̹۲쵽������Ϊ_____________________________��
��4����Ӧ���У��������������ҵ��Թܵ�����ˮ���У���ַ�Ӧ���Թ���Һ��ռ�Թ������_______________��
��5��ij��ƶѪ֢����Ӧ����C���ʵ������ӡ��������ӵ�ҩƬ�������һ����������£�������µ����þ��DZ��������Ӳ��������е�����������Ϊ���鳤�ڷ��õ�ҩƬ�Ѿ�ʧЧ����ҩƬȥ�����º����飬ȡ���������ҩƬ�����ձ��У�������������ˮ��Ȼ��μ�����__________��Һ����Һ��__________ɫ��������ҩƬ��ʧЧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F�������ʵ��ת����ϵ����ͼ��ʾ����Ӧ���������ֲ���δ�г�����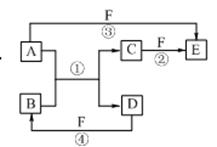
��1����A�dz����������ʣ���B��ˮ��Һ��Ӧ����C��D��D��F�����嵥�ʣ�D��F��ȼ��ʱ������ɫ���档��F����Ӧ��Ԫ�������ڱ�λ���� ����Ӧ�ڣ���ˮ��Һ�н��У������ӷ���ʽΪ ��
��2����A��DΪ������Ԫ����ɵĹ��嵥�ʣ�AΪ������DΪ�ǽ������Ңۢ�������Ӧ���к���ɫ�������ɣ���Ӧ�١��ܵĻ�ѧ����ʽ�ֱ�Ϊ
�� ���� ��
��3����A��D��F���Ƕ����ڷǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ�C��һ������Ѫ�쵰��ϵ��ж����壻������B�ľ��������� ������E�Ľṹʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ3�ֵ��ʣ�����AΪ���壬B��CΪ���壩����D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ���������ˮ�õ���ɫ��ҺE������֮���ת����ϵ����ͼ��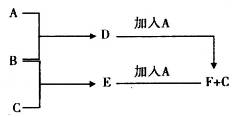
��д���пհף�
��1������A��____________������B��___________��BԪ�ص����ӽṹʾ��ͼΪ___________��
��2��д��������E�ĵ���ʽ��____________��D�ı�����Һ�����ˮ����Һ�ʺ��ɫ��ԭ���ǣ������ӷ���ʽ��ʾ����_______________________________��D��Һ��������ֹѪ��
��3����ҵ�ϰ�B������ʯ���鷴Ӧ���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���_______��Ư�۳���������ˮ��ɱ��������ԭ���ǣ����û�ѧ����ʽ���ʵ�������˵����______________________��
��4��F�м���NaOH��Һ�����ڿ����з��ã������ɰ�ɫ��Ϊ����ɫ����ɺ��ɫ�Ļ�ѧ����ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�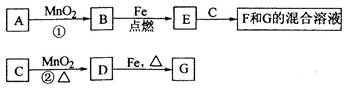
��ش��������⣺(����MnO2������������MnO2��������)
��1������C��E�����Ʒֱ�Ϊ________________��__________________��
��2����ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ_____________����ֻ���ڼ�������½��У���Ӧ��AӦΪ_____________��
��3����Ӧ�ڵĻ�ѧ����ʽ:_______________________________________��
��4�������Ƶ�F��ҺӦ����_____________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���_____________��ʵ������Ϊ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ���֣�A��B��C��D��E��X���±��и���Ԫ����ɵij������ʻ��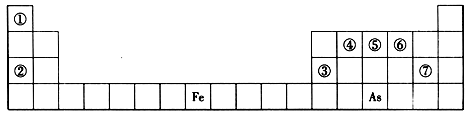
��֪A��B��C��D��E��X������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ��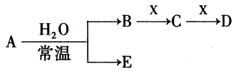
��1����EΪ�������壬DΪ��ɫ������A�Ļ�ѧʽ������ �� C��X��Ӧ�����ӷ���ʽΪ ��
��2����EΪ�������A��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
�ٵ�X�Ǽ�������Һ��C��������22������ʱ����C�ĽṹʽΪ ����ʾX�ʼ��Ե����ӷ���ʽΪ ��
�ڵ�XΪ��������ʱ��X��B��ϡ��Һ��Ӧ����C�����ӷ���ʽΪ ��
��3����BΪ�������壬D����ˮ������һ�������·������淴Ӧ������C��һ�ֿ�ȼ�����嵥�ʣ�д���ÿ��淴Ӧ�Ļ�ѧ����ʽ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ���ﵽƽ�⣬���¶��·�Ӧ��ƽ�ⳣ��K=1��D��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼ����ѧ��ѧ�г�������֮���һЩ��Ӧ��ϵ�����в��ֲ���δд����������X��H�ǹ��壬B��G��Һ�壬�����Ϊ���壬 1 mol X�ֽ�õ�A��B��C��1 mol��
�Իش����и��⣺
��1��д���������ʵĻ�ѧʽ��X________��B________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��H��G�D��A��F��__________________________________________________________��
��C��D�D��E��__________________________________________________________��
��3��д�����з�Ӧ�����ӷ���ʽ��
G��Cu�D��E��___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����и��仯�У�EΪ��ɫ��ζ��Һ�壨�����£���FΪ����ɫ��ĩ��GΪ��������ɫ���壨��Ӧ��������ʡ�ԣ���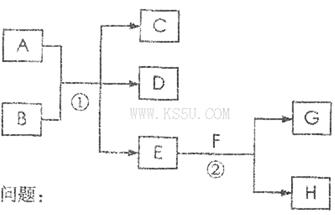
�ش��������⣺
��1���ڷ�Ӧ���У�ÿ����2.24L����G����״����ʱ���÷�Ӧת�Ƶ��ӵ����ʵ�����___________mol��
��2������Ӧ���ڼ��������½��У�����A�ͻ�����B�����ʵ���֮��Ϊ1��2������Ӧ����C��D�����־���ʹ�����ʯ��ˮ����ǵ���ɫ���壬��Ӧ�ٵĻ�ѧ����ʽ��___________________________________________��
��3������Ӧ������Һ�н��У�A��һԪǿ�B��һ����ʽ�Σ�D��һ��ʹʪ���ɫʯ����ֽ���������壬��B������������ʹƷ����Һ��ɫ�����塣�ڼ��������£���A����ʱ����Ӧ�ٵ����ӷ���ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���г�����������A��B��C�ͳ�������ס��ҡ���������D��E��F��G��H������֮���ܷ������·�Ӧ��ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ���������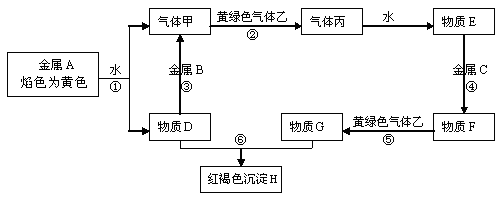
�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽ�� A ����
��2��д����Ӧ�ڵĻ�ѧ����ʽ
��3��д�����з�Ӧ���ӷ���ʽ��
��Ӧ��
��Ӧ��
��Ӧ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com