
 ��
�� ����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ��
����װ���ñ�������ϴ����25.00 mL��ʽ�ζ����У�����Һ��λ�� ����̶����£������¿̶ȣ�
����̶����£������¿̶ȣ� _____________________________��
_____________________________��| A����ʪ���pH��ֽ�ⶨijNaOH��Һ��pH |
| B���к͵ζ�ʵ����������ˮϴ������ƿֱ��װ����Һ |
| C�����ζ�ǰ��ʽ�ζ��ܼ�������δ�ų����ζ�������������ʧ |
D��װ������� ��ʽ�ζ���û�н�����ϴ ��ʽ�ζ���û�н�����ϴ |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B��NaOH��Һ | C������ | D��ʳ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
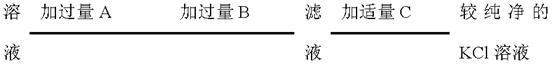

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������Ȼ�̼ | B��������Ҵ� | C�������ˮ | D������������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����ˮ�Ϳ�������Һ�е��κ�һ���Լ������ɼ����һ�������ǣ�������
����ˮ�Ϳ�������Һ�е��κ�һ���Լ������ɼ����һ�������ǣ�������A��NaCl��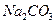 ���־��� ���־��� | B��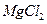 �� ��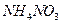 ������Һ ������Һ |
C��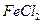 �� ��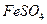 ������Һ ������Һ | D��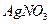 �� ��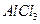 ������Һ ������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ù��յķ�����������ͭ��Һ�������������壻 |
| B����ϡ����������BaCl2�� NaCl�� Na2CO3������Һ�� |
| C����BaCl2��Һ��ϡ����������Na2SO4��AgNO3������Һ�� |
| D����CCl4������FeCl3��Һ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���� | B��ϡ���� |
| C����������Һ | D���Ȼ�þ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | �廯����Һ��NaI�� | ��ˮ��CCl4 | ��ȡ����Һ |
| B | CO��CO2�� | ����NaHCO3��Һ | ϴ�� |
| C | FeSO4��Fe2(SO4)3�� | ������м | ���� |
| D | CO2��SO2�� | NaOH | ϴ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com