
 ijŹµŃ銔×éÓĆ0.50mol/L NaOHČÜŅŗŗĶ0.50mol/L H2SO4ČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø£ŗ
ijŹµŃ銔×éÓĆ0.50mol/L NaOHČÜŅŗŗĶ0.50mol/L H2SO4ČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø£ŗ| Ćū³Ę | ĶŠÅĢĢģĘ½£Ø“ųķĄĀė£© | Š”ÉÕ± | ŪįŪöĒÆ | ²£Į§°ō | Ņ©³× | ĮæĶ² |
| ŅĒĘ÷ |  |  |  |  |  |  |
| ŠņŗÅ | a | b | c | d | e | f |
| ŹµŃé“ĪŹż ĪĀ¶Č | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | Ę½¾łĪĀ¶Č²ī £Øt2-t1£©/”ę | ||
| H2SO4 | NaOH | Ę½¾łÖµ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
·ÖĪö ¢ń£®£Ø1£©øł¾Ż¹«Ź½m=nM=cVMĄ“¼ĘĖćĒāŃõ»ÆÄʵÄÖŹĮ棬µ«ŹĒƻӊ245mLµÄČŻĮæĘ棻
£Ø2£©ĒāŃõ»ÆÄĘŅŖŌŚŠ”ÉÕ±ÖŠ³ĘĮ棬øł¾Ż³ĘĮæ¹ĢĢåĒāŃõ»ÆÄĘĖłÓƵÄŅĒĘ÷Ą“»Ų“š£»
¢ņ£®£Ø1£©øł¾ŻĖį¼īÖŠŗĶ·“Ӧɜ³É1molŅŗĢ¬Ė®Ź±·Å³ö57.3kJµÄČČĮæŹéŠ“ČČ»Æѧ·½³ĢŹ½£»
£Ø2£©¢ŁĻČÅŠ¶ĻĪĀ¶Č²īµÄÓŠŠ§ŠŌ£¬Č»ŗóĒó³öĪĀ¶Č²īĘ½¾łÖµ£»
¢ŚĻČøł¾ŻQ=m•c•”÷T¼ĘĖć·“Ó¦·Å³öµÄČČĮæ£¬Č»ŗóøł¾Ż”÷H=-$\frac{Q}{n}$kJ/mol¼ĘĖć³ö·“Ó¦ČČ£»
¢Ūa£®×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī£¬²āµĆµÄČČĮæĘ«Š”£»
b£®ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż£¬»įµ¼ÖĀĖłĮæµÄĒāŃõ»ÆÄĘĢå»żĘ«“󣬷ųöµÄČČĮæĘ«øߣ»
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ£¬²āµĆµÄČČĮæĘ«Š”£»
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č£¬ĮņĖįµÄĘšŹ¼ĪĀ¶ČĘ«øߣ®
½ā“š ½ā£ŗ¢ń£®£Ø1£©Ć»ÓŠ245mLµÄČŻĮæĘ棬ĖłŅŌÓĆ250mLµÄČŻĮæĘ棬ŠčŅŖ³ĘĮæNaOH¹ĢĢåm=nM=cVM=0.5mol/L”Į0.25L”Į40g/mol=5.0g£¬
¹Ź“š°øĪŖ£ŗ5.0£»
£Ø2£©ĒāŃõ»ÆÄĘŅŖŌŚŠ”ÉÕ±ÖŠ³ĘĮ棬³ĘĮæ¹ĢĢåĒāŃõ»ÆÄĘĖłÓƵÄŅĒĘ÷ÓŠĢģĘ½”¢ÉÕ±ŗĶŅ©³×£¬
¹Ź“š°øĪŖ£ŗa b e£»
¢ņ£®£Ø1£©ŅŃÖŖĻ”ĒæĖį”¢Ļ”Ēæ¼ī·“Ӧɜ³É1molŅŗĢ¬Ė®Ź±·Å³ö57.3kJµÄČČĮ棬Ļ”ĮņĖįŗĶĒāŃõ»Æ±µÄĘĻ”ČÜŅŗ¶¼ŹĒĒæĖįŗĶĒæ¼īµÄĻ”ČÜŅŗ£¬Ōņ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ$\frac{1}{2}$H2SO4£Øaq£©+NaOH£Øaq£©=$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©”÷H=-57.3kJ/mol£¬
¹Ź“š°øĪŖ£ŗ$\frac{1}{2}$H2SO4£Øaq£©+NaOH£Øaq£©=$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©”÷H=-57.3kJ/mol£»
£Ø2£©4“ĪĪĀ¶Č²ī·Ö±šĪŖ£ŗ4.0”ę£¬4.1”ę£¬3.9”ę£¬4.1”ę£¬4×鏿¾Ż¶¼ÓŠŠ§£¬ĪĀ¶Č²īĘ½¾łÖµ=$\frac{4.0”ę+4.1”ę+3.9”ę+4.1”ę}{4}$=4.0”ę£¬
¹Ź“š°øĪŖ£ŗ4.0£»
¢Ś50mL0.50mol/LĒāŃõ»ÆÄĘÓė30mL0.50mol/LĮņĖįČÜŅŗ½ųŠŠÖŠŗĶ·“Ӧɜ³ÉĖ®µÄĪļÖŹµÄĮæĪŖ0.05L”Į0.50mol/L=0.025mol£¬ČÜŅŗµÄÖŹĮæĪŖ£ŗ80ml”Į1g/ml=80g£¬ĪĀ¶Č±ä»ÆµÄÖµĪŖ”÷T=4”ę£¬ŌņÉś³É0.025molĖ®·Å³öµÄČČĮæĪŖQ=m•c•”÷T=80g”Į4.18J/£Øg•”ę£©”Į4.0”ę=1337.6J£¬¼“1.3376KJ£¬ĖłŅŌŹµŃé²āµĆµÄÖŠŗĶČČ”÷H=-$\frac{1.3376kJ}{0.025mol}$=-53.5 kJ/mol£¬
¹Ź“š°øĪŖ£ŗ-53.5kJ/mol£»
¢Ūa£®×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī£¬²āµĆµÄČČĮæĘ«Š”£¬ÖŠŗĶČȵďżÖµĘ«Š”£¬¹ŹaÕżČ·£»
b£®ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż£¬»įµ¼ÖĀĖłĮæµÄĒāŃõ»ÆÄĘĢå»żĘ«“󣬷ųöµÄČČĮæĘ«øߣ¬ÖŠŗĶČȵďżÖµĘ«“󣬹Źb“ķĪó£»
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ£¬ČČĮæÉ¢Ź§£¬ÖŠŗĶČȵďżÖµĘ«Š”£¬¹ŹcÕżČ·£»
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č£¬ĮņĖįµÄĘšŹ¼ĪĀ¶ČĘ«øߣ¬ĪĀ¶Č²īĘ«Š”£¬²āµĆµÄČČĮæĘ«Š”£¬ÖŠŗĶČȵďżÖµĘ«Š”£¬¹ŹdÕżČ·£»
¹Ź“š°øĪŖ£ŗacd£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éÖŠŗĶČČµÄ²ā¶Ø£¬ĢāÄæÄѶČÖŠµČ£¬Éę¼°ČÜŅŗµÄÅäÖĘ”¢ČČ»Æѧ·½³ĢŹ½ŅŌ¼°·“Ó¦ČČµÄ¼ĘĖć£¬×¢ŅāĄķ½āÖŠŗĶČȵÄøÅÄī”¢°ŃĪÕČČ»Æѧ·½³ĢŹ½µÄŹéŠ“·½·Ø£¬ŅŌ¼°²ā¶Ø·“Ó¦ČȵÄĪó²īµČĪŹĢā£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪöÄÜĮ¦¼°»ÆѧŹµŃéÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µ±»ģŗĻĘųĢåµÄÖŹĮæ²»±ä£¬ĖµĆ÷·“Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬ | |
| B£® | ĖõŠ”ČŻĘ÷Ģå»ż£¬ÖŲŠĀ“ļµ½Ę½ŗāŹ±£¬ĘųĢåĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»±ä»ņŌö“ó | |
| C£® | ŌŚŗćŃ¹ČŻĘ÷ÖŠÉżøßĪĀ¶Č£¬ĘųĢå»ģŗĻĪļÖŠC%æÉÄÜĻČŌö“óŗó¼õŠ” | |
| D£® | ŌŚŗćČŻČŻĘ÷ÖŠÉżøßĪĀ¶Č£¬“ļµ½ŠĀĘ½ŗāŹ±£¬ĘųĢåµÄĆܶČæÉÄÜŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
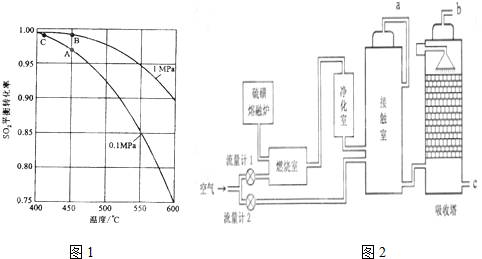
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1minÄŚÓĆO2±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ0.03 mol•L-1•min-1 | |
| B£® | øĆ·“Ó¦µÄĘ½ŗā³£ŹżŹżÖµĪŖ7.5 | |
| C£® | SO2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ60% | |
| D£® | ½µµĶĪĀ¶Č£¬SO2Ę½ŗāÅØ¶Č¼õŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
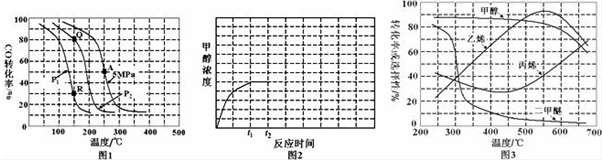
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
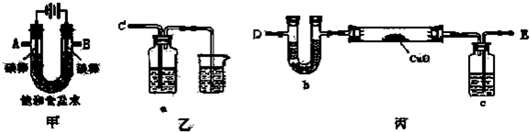
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢĄÄ·Éś | B£® | ²£¶ū | C£® | Ā¬ÉŖø£ | D£® | ĘÕĄŹæĖ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com