
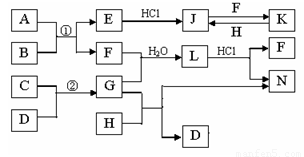
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ�٢��ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ��������ˮ����Һ��.
��1��������A�к��еĶ�����Ԫ���� ��дԪ�ط��ţ���E�Ļ�ѧʽ_______��
��2����μ��黯����N�е������� ��
��3��д��J��F��Ӧ�����ӷ���ʽ�� ��G��H��Ӧ�Ļ�ѧ����ʽ�� ��
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��ԭ���ǣ��û�ѧ��Ӧ����ʽ��ʾ�� ��
��5����֪C��D��Ӧ����1.7g G ʱ�ų�4.26kJ����������÷�Ӧ���Ȼ�ѧ����Ϊ
��
��1��S ��2�֣� ��Fe2O3��1�֣�
��2��ȡ������Ʒ���Թ��У���ˮ�ܽ⣬�μ�NaOHŨ��Һ����ȣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڸ�������ֽ��������˵����NH4+��2�֣�
��3��2Fe3++ Fe =3Fe2+��2�֣� �� 8NH3+3Cl2=6NH4Cl+N2��2�֣�
��4��Ʒ����Һ��ɫ��2�֣� �� SO2+Cl2+2H2O=H2SO4+2HCl��2�֣�
��5��N2(g)+3H2(g)=2NH3(g) ��H=-85.2kJ/mol��2�֣���
����:��


 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ�٢��ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ��������ˮ����Һ��.
��1��������A�к��еĶ�����Ԫ���� ��дԪ�ط��ţ���E�Ļ�ѧʽ_______��
��2����μ��黯����N�е������� ��
��3��д��J��F��Ӧ�����ӷ���ʽ�� ��G��H��Ӧ�Ļ�ѧ����ʽ�� ��
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��ԭ���ǣ��û�ѧ��Ӧ����ʽ��ʾ�� ��
��5����֪C��D��Ӧ����1.7g G ʱ�ų�4.26kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����������һ�и����������¿��������ۺϣ���ѧ�� ���ͣ������
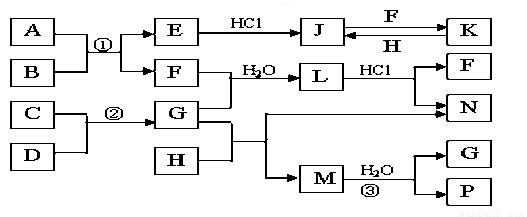
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ���ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��P ��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ�������������������M������Ԫ����ɣ������ڹ���58�����ӡ�
��1��������A�к��е�����Ԫ���� ��дԪ�ط��ţ���2�֣�M�Ļ�ѧʽ_______��1�֣�
��2����μ��黯����N�е������� ��2�֣�
��3��д��K��H��Ӧ�����ӷ���ʽ�� ��2�֣�
C��D��Ӧ�Ļ�ѧ����ʽ�� ��2�֣�
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��2�֣���ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ�� ��2�֣�
��5��ʵ���п���NaOH��Һ�����ն����H����д�������ӷ�Ӧ����ʽ
(2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�������Ĵ��¿��������ۺϣ���ѧ���� ���ͣ������
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ�٢��ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ��������ˮ����Һ��.
��1��������A�к��еĶ�����Ԫ���� ��дԪ�ط��ţ���E�Ļ�ѧʽ_______��
��2����μ��黯����N�е������� ��
��3��д��J��F��Ӧ�����ӷ���ʽ�� ��G��H��Ӧ�Ļ�ѧ����ʽ�� ��
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��ԭ���ǣ��û�ѧ��Ӧ����ʽ��ʾ�� ��
��5����֪C��D��Ӧ����1.7g G ʱ�ų�4.26kJ����������÷�Ӧ���Ȼ�ѧ����Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ĸ����������¿��������ۺϣ���ѧ�� ���ͣ������
��ͼ�Dz��ֳ���Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵIJ��ֲ�������ȥ������֪��EΪ����ɫ���壬KΪdz��ɫ��Һ����Ӧ���ǻ��������е���Ҫ��Ӧ��B��C��D��H�ǵ��ʣ�B��C��D��F��G��H����������̬�� F��P ��H��ˮ��Һ������Ư�����ã���F���γ��������Ҫ����֮һ��N��һ�ֳ����ĵ��ʣ�������GΪ�������������������M������Ԫ����ɣ������ڹ���58�����ӡ�
��1��������A�к��е�����Ԫ���� ��дԪ�ط��ţ���2�֣�M�Ļ�ѧʽ_______��1�֣�
��2����μ��黯����N�е������� ��2�֣�
��3��д��K��H��Ӧ�����ӷ���ʽ�� ��2�֣�
C��D��Ӧ�Ļ�ѧ����ʽ�� ��2�֣�
��4�������ʵ���F��H�Ļ������ͨ��Ʒ����Һ�е�����Ϊ ��2�֣���ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ�� ��2�֣�
��5��ʵ���п���NaOH��Һ�����ն����H����д�������ӷ�Ӧ����ʽ
(2�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com