
 Ē”ŗĆĶźČ«·“Ó¦”£Bµ„ÖŹÓėDµ„ÖŹ·“Ó¦ŗóæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļ
Ē”ŗĆĶźČ«·“Ó¦”£Bµ„ÖŹÓėDµ„ÖŹ·“Ó¦ŗóæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļ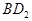 £»Bµ„ÖŹÓėEµ„ÖŹæÉŠĪ³É»ÆŗĻĪļBE”£DµÄŅõĄė×Ó±ČBµÄŃōĄė×Ó¶ąŅ»øöµē×Ó²ć£¬¶ųEŅõĄė×ÓÓėBµÄŃōĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬”£
£»Bµ„ÖŹÓėEµ„ÖŹæÉŠĪ³É»ÆŗĻĪļBE”£DµÄŅõĄė×Ó±ČBµÄŃōĄė×Ó¶ąŅ»øöµē×Ó²ć£¬¶ųEŅõĄė×ÓÓėBµÄŃōĄė×Óµē×Ó²ć½į¹¹ĻąĶ¬”£ µÄ»Æѧ¼üĄąŠĶŹĒ£ŗ £®
µÄ»Æѧ¼üĄąŠĶŹĒ£ŗ £® £Ø2·Ö£©
£Ø2·Ö£©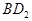 £¬ĖµĆ÷BŹĒµŚ¢ņA£¬DŹĒµŚ¢÷A”£2gBµÄŃõ»ÆĪļÓė100ml 0.5mol/LµÄ
£¬ĖµĆ÷BŹĒµŚ¢ņA£¬DŹĒµŚ¢÷A”£2gBµÄŃõ»ÆĪļÓė100ml 0.5mol/LµÄ Ē”ŗĆĶźČ«·“Ó¦£¬ĖłŅŌBµÄŃõ»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ40£¬Ņņ“ĖBŹĒĆ¾£¬ŌņAŹĒNa£¬CŹĒAl£¬DŹĒCl£¬EŹĒO”£
Ē”ŗĆĶźČ«·“Ó¦£¬ĖłŅŌBµÄŃõ»ÆĪļµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒ40£¬Ņņ“ĖBŹĒĆ¾£¬ŌņAŹĒNa£¬CŹĒAl£¬DŹĒCl£¬EŹĒO”£ ”£
ӣ


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŖĖŲ·Ē½šŹōŠŌÓÉĒæµ½ČõµÄĖ³ŠņŹĒ£ŗ Z> Y>X |
| B£®YŌŖĖŲ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄ»ÆѧŹ½æɱķŹ¾ĪŖH4YO4 |
| C£®3ÖÖŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļÖŠ£¬ZµÄĘųĢ¬Ēā»ÆĪļ×īĪČ¶Ø |
| D£®Ō×Ó°ė¾¶Óɓ󵽊”µÄĖ³ŠņĪŖ Z >Y>X |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®·Ö×ÓÖŠÖŠŠÄŌ×ÓĶعżSP3ŌӻƹģµĄ³É¼üŹ±£¬øĆ·Ö×Ó²»Ņ»¶ØĪŖÕżĖÄĆęĢå½į¹¹ |
| B£®ŌӻƹģµĄÖ»ÓĆÓŚŠĪ³É¦Ņ¼ü»ņÓĆÓŚČŻÄÉĪ“²ĪÓė³É¼üµÄ¹Āµē×Ó¶Ō |
| C£®ŅŅĖį·Ö×ÓÖŠČżÖÖŌ×Ó¾łŅŌŌӻƹģµĄ³É¼ü |
| D£®N2·Ö×ÓÖŠNŌ×ÓƻӊŌӻƣ¬·Ö×ÓÖŠÓŠŅ»øö¦Ņ¼ü”¢2øö¦Š¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| ŌŖĖŲ±ąŗÅ ŌŖĖŲŠŌÖŹ | ¢Ł | ¢Ś | ¢Ū | ¢Ü | ¢Ż | ¢Ž | ¢ß |
| Ō×Ó°ė¾¶£Ø10£10m£© | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 |
| ×īøßÕż»ÆŗĻ¼Ū | | +2 | +1 | +5 | +7 | +1 | +5 |
| ×īµĶøŗ»ÆŗĻ¼Ū | £2 | | | £3 | £1 | | £3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®RŅ»¶ØŹĒµŚĖÄÖÜĘŚŌŖĖŲ |
| B£®RµÄĘųĢ¬Ēā»ÆĪļ±ČĶ¬ÖÜĘŚĘäĖūŌŖĖŲĘųĢ¬Ēā»ÆĪļĪČ¶Ø |
| C£®RŅ»¶ØŹĒ¢ōA×åŌŖĖŲ |
| D£®RĘųĢ¬Ēā»ÆĪļ»ÆѧŹ½ĪŖH2R |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼īŠŌ£ŗNaOH>Mg(OH)2 | B£®Ō×Ó°ė¾¶£ŗCl>S |
| C£®½šŹōŠŌ£ŗK>Na | D£®ČČĪČ¶ØŠŌ£ŗNH3>PH3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com