
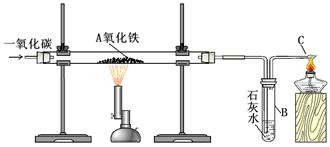
 2Fe3O4+CO2
2Fe3O4+CO2  4Fe+4CO2
4Fe+4CO2 | ʵ����� | Ԥ������ͽ��� |
| ����һ��ȡӲ�ʲ������й�����������ֱ���A��B�Թ��У���������1mol/LCuSO4��Һ�������ܽ⡣ | ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����Ϊ ��2����B�Թ����к�ɫ������������˵����ɫ�����к���Fe�� |
| ����������Թ�B����Һ���ˣ������ù���ϴ�Ӹɾ�������1mol/L����������ηֱ��������0.01mol/L��ˮ������0.01mol/L KSCN��Һ | ��1������Һ�����ɫ���� ��2������Һ���ɫ���� |
| ʵ����� | Ԥ������ͽ��� |
| | ��1����A�Թ��к�ɫ���岻�ܽ⣬����û�й۲쵽�����������ɫ����ΪFe3O4��1�֣� |
| | ��1������Һ�����ɫ��������1��ȷ ��2�֣� ��2������Һ���ɫ��������3��ȷ��2�֣� |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ױ����ұ� | B���������������顢�ȱ� |
| C���Ȼ��ơ��Ȼ��ء��Ȼ�ͭ | D��̼�����ơ��Ȼ��ơ��Ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ����Ư��������������ˮ����ֽ��裬���ã����ˣ��ó�������Һ�� | |
| ����2���������������2mol��L��1HCl��Һ��������������ͨ�� | ���� ���ۣ� |
| ����3��ȡ��Һ��װA��B��֧�Թܡ���A�Թܣ� | ������Һ�ȱ��ɫ��Ȼ����ɫ�� ���ۣ� |
| ����4����B�Թܣ� | ��������ɫ������ ���ۣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
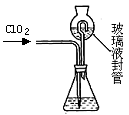
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
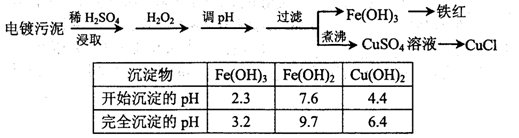
 Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ��Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ����� | �Լ� | ���뷽�� |
| A | ����(������) | ������Һ | ���� |
| B | ����(��ϩ�� | ��ˮ | ϴ�� |
| C | ��������(����) | ����������Һ | ���� |
| D | ���۽���(�Ȼ�����Һ) | ����ˮ | ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢ� | B���٢ۢݢڢ� | C���ڢݢ٢ۢ� | D���ڢ٢ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2S | B��Cl2 |
| C��HCl | D��CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com