
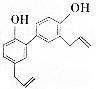
 ÖŠŅ©ŗńĘÓĘ¤ÖŠĘšæ¹¾ś×÷ÓƵÄÓŠŠ§³É·ÖĪŖŗńĘÓ·Ó£¬Ęä½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£®øĆĪļÖŹ¾ßÓŠ³Ö¾ĆµÄ¼”ČāĖɳŚ×÷ÓĆŗĶ¼«ĒæµÄæ¹¾ś×÷ÓĆ£¬ĮŁ“²ÉĻÖ÷ŅŖÓĆÓŚÕņ¾²ÖŠŹąÉń¾”¢æ¹Õę¾śµČ£®
ÖŠŅ©ŗńĘÓĘ¤ÖŠĘšæ¹¾ś×÷ÓƵÄÓŠŠ§³É·ÖĪŖŗńĘÓ·Ó£¬Ęä½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£®øĆĪļÖŹ¾ßÓŠ³Ö¾ĆµÄ¼”ČāĖɳŚ×÷ÓĆŗĶ¼«ĒæµÄæ¹¾ś×÷ÓĆ£¬ĮŁ“²ÉĻÖ÷ŅŖÓĆÓŚÕņ¾²ÖŠŹąÉń¾”¢æ¹Õę¾śµČ£®| A£® | 1øöŗńĘÓ·Ó·Ö×ÓÖŠŗ¬ÓŠ16øöĢ¼Ō×Ó | |
| B£® | ŗńĘÓ·ÓÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬ÓöFeCl3ČÜŅŗĻŌÉ« | |
| C£® | 1molŗńĘÓ·ÓÓė×ćĮæNaHCO3ČÜŅŗ·“Ó¦·Å³ö22.4LCO2£Ø±ź×¼×“æö£© | |
| D£® | 1molŗńĘÓ·ÓÄÜÓė4molBr2£ØBr2µÄCCl4ČÜŅŗ£©·“Ó¦ |
·ÖĪö ÓŠ»śĪļŗ¬ÓŠ·ÓōĒ»ł£¬æÉ·¢ÉśČ”“ś”¢Ńõ»Æ·“Ó¦£¬ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬æÉ·¢Éś¼Ó³É”¢¼Ó¾ŪŗĶŃõ»Æ·“Ó¦£¬½įŗĻ±½»·ŗĶĢ¼Ģ¼Ė«¼üµÄ½į¹¹ĢŲµć½ā“šøĆĢā£®
½ā“š ½ā£ŗA£®Óɽį¹¹¼ņŹ½æÉÖŖÓŠ»śĪļŗ¬ÓŠĢ¼Ō×ÓŹżĪŖ18øö£¬¹ŹA“ķĪó£»
B£®ŗ¬ÓŠ·ÓōĒ»ł£¬æÉÓėFeCl3ČÜŅŗ·¢ÉśŃÕÉ«·“Ó¦£¬ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬æɱ»ĖįŠŌøßĆĢĖį¼ŲŃõ»Æ£¬¹ŹBÕżČ·£»
C£®·ÓōĒ»łµÄĖįŠŌ±ČĢ¼ĖįČõ£¬ÓėĢ¼ĖįĒāÄĘ²»·“Ó¦£¬¹ŹC“ķĪó£»
D£®Ö»ÓŠĢ¼Ģ¼Ė«¼üÓėBr2µÄCCl4ČÜŅŗ·¢Éś¼Ó³É·“Ó¦£¬¶ų·ÓōĒ»łÓėäåĖ®·¢ÉśČ”“ś·“Ó¦£¬Ōņ1molŗńĘÓ·ÓÄÜÓė2molBr2£ØBr2µÄCCl4ČÜŅŗ£©·“Ó¦£¬¹ŹD“ķĪó£®
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄ½į¹¹ÓėŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕ¹ŁÄÜĶÅÓėŠŌÖŹµÄ¹ŲĻµĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲ·Ó”¢Ļ©ĢžŠŌÖŹµÄæ¼²é£¬Ń”ĻīDĪŖ½ā“šµÄÄŃµć£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ČēĶ¼ĖłŹ¾£¬ŅŃÖŖAµÄ²śĮæŹĒŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤Ė®Ę½µÄ±źÖ¾£¬D¾ßÓŠĖįŠŌ£®EŹĒ¾ßÓŠĻćĪ¶µÄ²»ČÜÓŚĖ®µÄŅŗĢ壮
ČēĶ¼ĖłŹ¾£¬ŅŃÖŖAµÄ²śĮæŹĒŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤Ė®Ę½µÄ±źÖ¾£¬D¾ßÓŠĖįŠŌ£®EŹĒ¾ßÓŠĻćĪ¶µÄ²»ČÜÓŚĖ®µÄŅŗĢ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
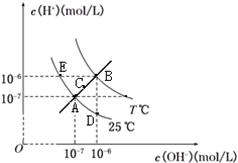
| A£® | Ķ¼ÖŠĪåµćKW¼ä¹ŲĻµ£ŗB£¾C£¾A=D=E | |
| B£® | Eµć¶ŌÓ¦µÄĖ®ČÜŅŗÖŠ£¬æÉÄÜÓŠNH${\;}_{4}^{+}$”¢Ba2+”¢Cl-”¢I-”¢K“óĮæĶ¬Ź±“ęŌŚ | |
| C£® | Čō“¦ŌŚBµćŹ±£¬½«pH=3µÄĮņĖįČÜŅŗÓėpH=9µÄKOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗ³ŹÖŠŠŌ | |
| D£® | Čō0.1mol/LµÄNaHBČÜŅŗÖŠc£ØOH+£©Óėc£ØOH-£©¹ŲĻµČēĶ¼DµćĖłŹ¾£¬ŌņČÜŅŗÖŠÓŠ£ŗc£ØHB-£©£¾c£ØOH-£©£¾c£ØB2-£©£¾c£ØH2B£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ąė×Ó°ė¾¶£ŗNa+£¼S2-£¼Cl-£¼K+ | B£® | »¹ŌŠŌ£ŗI-£¼Br-£¼-Cl£¼F- | ||
| C£® | ĪČ¶ØŠŌ£ŗSiH4£¼PH3£¼HCl£¼HBr | D£® | ČܽāŠŌ£ŗBaO4£¼CaSO4£¼MgSO4£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
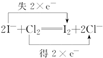 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
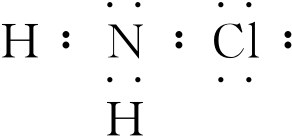 £¬H2NClÖŠĀȵĻÆŗĻ¼ŪĪŖ+1¼Ū£®
£¬H2NClÖŠĀȵĻÆŗĻ¼ŪĪŖ+1¼Ū£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com