
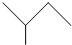
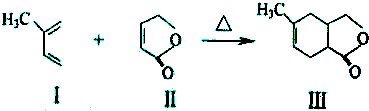
 ��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ
��CH��CH����Ȳ��Ҳ�ܷ���Diels-Alder��Ӧ����д���÷�Ӧ����Ľṹ��ʽ ��̼̼˫��������һ��̼̼�����ѣ��м��γ�1���µ�̼̼˫����CH��CH������һ��̼̼�����ѣ������ϼ��õ�̼ԭ��������γ�̼̼����
��̼̼˫��������һ��̼̼�����ѣ��м��γ�1���µ�̼̼˫����CH��CH������һ��̼̼�����ѣ������ϼ��õ�̼ԭ��������γ�̼̼���� ��
��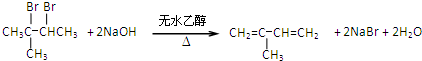 ��
��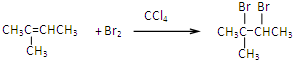 ��
�� ��
��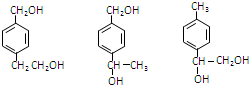 ��
�� ����һ�֣�
����һ�֣� ��̼̼˫��������һ��̼̼�����ѣ��м��γ�1���µ�̼̼˫����CH��CH������һ��̼̼�����ѣ������ϼ��õ�̼ԭ��������γ�̼̼������Ӧ����Ľṹ��ʽΪ��
��̼̼˫��������һ��̼̼�����ѣ��м��γ�1���µ�̼̼˫����CH��CH������һ��̼̼�����ѣ������ϼ��õ�̼ԭ��������γ�̼̼������Ӧ����Ľṹ��ʽΪ��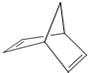 ��
�� ��
��


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
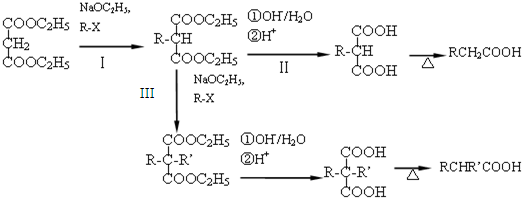
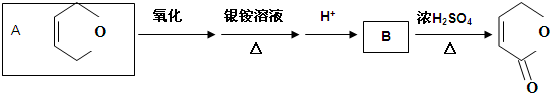
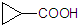 ������������Ľṹ��ʽΪ
������������Ľṹ��ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��C2H2 |
| B��CO2 |
| C��NH3 |
| D��BF3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D����ǰ������Ԫ����ɵĵ��ʻ������֮����������ת����ϵ��
A��B��C��D����ǰ������Ԫ����ɵĵ��ʻ������֮����������ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
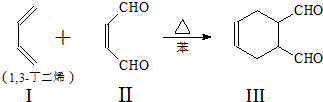
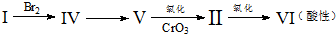
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
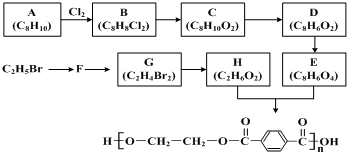
| �Լ�a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� | ������Һ�����ml�� | �����������ml�� | |
| �ζ�ǰ�̶ȣ�ml�� | �ζ���̶ȣ�ml�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 0.20 | 20.80 |
| ������ | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��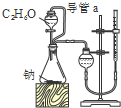 �ⶨ�Ҵ��ṹʽ |
B��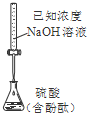 �ⶨ����Ũ�� |
C��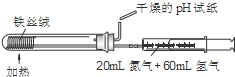 �ϳɲ����鰱 |
D��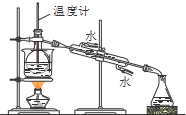 ���벢�����������еı� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com