
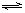 2SO3��g�����ﵽƽ��ʱ��SO3���������Ϊ0.91��
2SO3��g�����ﵽƽ��ʱ��SO3���������Ϊ0.91�� ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 4V |
| 3 |
| 4V |
| 3 |
| 4V |
| 3 |
| 4V |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���������Ͽ���ѹǿ����ƽ��������Ӧ�����ƶ���SO2��ת����������ҵ�϶���������������ó�ѹ�����ø�ѹ����Ҫ��ԭ����____________________________��
��2�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2 mol SO3������������������ƽ��ʱSO2�����������________����ʱƽ��������������________��
��3���¶��Ա���537 �棬�������������200 L���䡣����a mol SO2��b mol O2������������������Ӧ�ﵽƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ1.01��105 Pa����a��b=2��1����a=________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����������ѧ�߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
��8�֣���537�桢101KPa ʱ�����ݻ��ɱ���ܱ������г���2mol SO2�� 1mol O2����ʱ���������Ϊ200L���������м�����������壩�����ֺ��º�ѹ��������Ӧ��2SO2(g)+O2(g)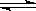 2SO3(g)�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ91%���Իش��������⣺
2SO3(g)�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ91%���Իش��������⣺
��1�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2mol SO3����������������ƽ��ʱ��SO2����������� �����������Ϊ L��
��2�����¶Ⱥ��������ݻ����䣬��ƽ��ʱ�������������ѹǿΪ KPa��
��3���¶��Ա���537�棬�����������200L���䣨���ݣ�������a molSO2��b molO2������������������Ӧ��ƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ101KPa����a:b=2:1����a= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ��У2009-2010ѧ��ȸ�����һ����������ѧ������ ���ͣ������
��537�桢1.01��105 Paʱ�����ݻ��ɱ���ܱ������г���1 mol X��3 mol Y����ʱ�ݻ�ΪV L�����ֺ��º�ѹ��������ӦX(g)+3Y(g)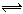 2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
��1���ﵽƽ��ʱ��X��ת����ԼΪ ��
��2�����������¶Ⱥ�ѹǿ�㶨���䣬����������ֻ����4 mol Z����Ӧ�ﵽƽ��ʱ��ƽ��������Y ���������Ϊ ���������ݻ�Ϊ L��
��3������ѡһ�ݻ��̶�������ܱ��������Կ����¶Ȳ��䣬ʹ2 mol X��6 mol Y��Ӧ���ﵽƽ��ʱƽ��������Z�����������Ϊ0.5������ܱ��������ݻ�Ϊ ��
��4�����¶���Ϊ537�棬�����������VL����(����)�������г���a mol X��b mol Y��ʹ��Ӧ�ﵽƽ�⣬��ʱƽ��������Z�����������Ϊ0.5����ϵѹǿΪ1.01��105 Pa����a: b = 1: 3����a = ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com