
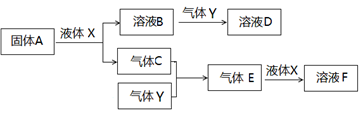
ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W������Ԫ�ص�ԭ������֮��Ϊ32�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ�Y��Z�������ڣ�Z��Wλ��ͬ���塣
��1��XԪ���� �������ƣ� ,W�����ڱ��е�λ�� ��
��2��X��Y �γɻ�����ĵ���ʽΪ ��X��W��ɵĻ������д��� ��������ӡ������ۡ�����
��3����д��ʵ�����Ʊ�YX3�Ļ�ѧ����ʽ�� ��
�ڹ�ҵ��Ҳ����ѡ����ʵ���������YX3�ĺϳɣ�����֪�ڸ�������ÿ����2molYX3����ʱ�ų�
92.4kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��4����X��Y��Z��W����Ԫ����ɵ�һ�����ӻ�����A����֪1mol A��������NaOHŨ��Һ��Ӧ���ɱ�״����44.8L���塣��A�������� ��
��1��H �������ڵڢ�A��
��2��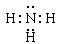 ����
����
��3����2NH4Cl + Ca��OH��2 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O
��N2(g)+3H2(g) 2NH3(g) ��H=-92.4KJ/mol
2NH3(g) ��H=-92.4KJ/mol
��4������炙��������
�������������ԭ��������С�������е����ֶ�����Ԫ��X��Y��Z��W�������ڱ���X��ԭ�Ӱ뾶��С��Ԫ�أ���XΪHԪ�أ�Z��Wλ��ͬ���壬��Z��ԭ������Ϊx����W��ԭ������Ϊx+8��Y��Z�������ڣ�Y��ԭ������Ϊx-1��������Ԫ�ص�ԭ������֮��Ϊ32����1+��x-1��+x+��x+8����32�����x��8����YΪNԪ�أ�ZΪOԪ�أ�WΪSԪ�ء�
��1��������������֪��XԪ����HԪ�أ�W����Ԫ�أ�λ��Ԫ�����ڱ��ĵ������ڵڢ�A�壬�ʴ�Ϊ��H���������ڵڢ�A�塣
��2��X��Y �γɵĻ������ǰ��������м��Լ��Ĺ��ۻ��������ʽΪ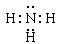 ��X��W��ɵĻ�������H2S��������H��S֮���Թ��õ��Ӷ��γɻ�������Թ��ۼ���ϣ��ʴ�Ϊ�����ۣ�
��X��W��ɵĻ�������H2S��������H��S֮���Թ��õ��Ӷ��γɻ�������Թ��ۼ���ϣ��ʴ�Ϊ�����ۣ�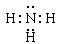 ��
��
��3����ʵ�����Ʊ�����������ʯ�Һ��Ȼ�識��ȣ���Ӧ�Ļ�ѧ����ʽΪ2NH4Cl + Ca��OH��2 CaCl2 + 2NH3��+ 2H2O��
CaCl2 + 2NH3��+ 2H2O��
��ÿ����2mol��������ʱ�ų�92.4kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g) 2NH3(g) ��H����92.4KJ/mol��
2NH3(g) ��H����92.4KJ/mol��
��4��X��Y��Z��W����Ԫ����ɵ�һ�����ӻ������е�������ΪNH4+��������Ϊ��������ӡ�����������ӡ�����������ӡ�������������ӣ���1mol A��������NaOHŨ��Һ��Ӧ���ɱ�״����44.8L���壬��1molA�к���2molNH4+����AΪ����炙�������李�
���㣺����ṹ��λ�ù�ϵ��Ԫ�ػ���������ʡ����û�ѧ�����


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣������������ʵ���Ҫ���������ʽṹ����ش��������⣺
��1��ͭ�ǹ���Ԫ�ء��������У�ͭ������+1�ۻ�+2�ۡ���ͼΪijͭ�����ᄃ��ṹ��Ԫ����������Ļ�ѧʽΪ ��
��2���������ڲ���Ԫ�ط�������۵���±���
| ������ | NaF | MgF2 | SiF4 |
| �۵�/K | 1266 | 1534 | 183 |
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
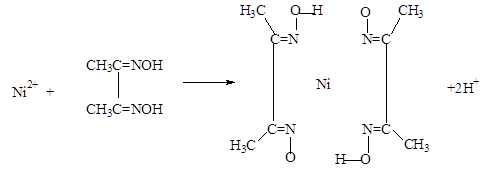
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��Ԫ�صĵ�һ������Al������Si���>����<������
��2����̬Mn2+�ĺ�������Ų�ʽΪ����������
��3�����飨SinH2n+2���ķе�������Է��������ı仯��ϵ��ͼ��ʾ,�������ֱ仯��ϵ��ԭ���� ��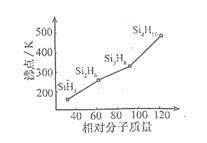
��4����ɰ�Ǻ��ᾧˮ���������ƣ���������Xm-����B��O��H����Ԫ�أ������ģ����ͼ��ʾ��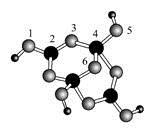
����Xm-��,��ԭ�ӹ�����ӻ�����������������λ������������ԭ��֮�䣨��ԭ�ӵ����ֱ�ţ���m=�������������֣���
����ɰ������Na+��Xm-��H2O���ɣ�����֮����ڵ���������������������ţ���
A�����Ӽ� B�����ۼ� C�������� D�����»�����E�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣��±�ΪԪ�����ڱ���һ���֣�
 �� ������ | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
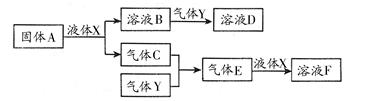
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о����ʵ��۽ṹ�������������������ʱ仯�ı��ʡ���ش��������⣺
(1)C��Si��NԪ�صĵ縺���ɴ�С��˳���� ��
C60�ͽ��ʯ����̼��ͬ�������壬������ȣ��۵�ߵ��� ��ԭ���� ��
(2)A��B��Ϊ�����ڽ���Ԫ�أ����ݱ������ݣ�д��B�Ļ�̬ԭ�ӵĵ����Ų�ʽ�� ��
| ������/(kJ��mol��1) | I1 | I2 | I3 | I4 |
| A | 932 | 1 821 | 15 390 | 21 771 |
| B | 738 | 1 451 | 7 733 | 10 540 |
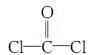 ��ÿ��COCl2�����ں��� ���Ҽ��� ���м���������ԭ�Ӳ�ȡ �ӻ������ʽ��
��ÿ��COCl2�����ں��� ���Ҽ��� ���м���������ԭ�Ӳ�ȡ �ӻ������ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣�
| �� ���� | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧԭ�Ӱ뾶�����ݣ�
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| ԭ�Ӱ뾶(nm) | 0.102 | 0.110 | 0.117 | 0.074 | 0.075 | 0.071 | 0.099 | 0.077 |
| ����ϼ� | ��6 | ��5 | ��4 | | ��5 | | ��7 | ��4 |
| ��ͻ��ϼ� | ��2 | ��3 | ��4 | ��2 | ��3 | ��1 | ��1 | ��4 |
 2AD3 ��H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ��� (����ӡ������١����䡱)��ԭ��Ϊ ��
2AD3 ��H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ��� (����ӡ������١����䡱)��ԭ��Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֶ�����Ԫ��A��B��C��D��E��ԭ��������������A��Cͬ�壬B��Dͬ�壬C���Ӻ�B���Ӿ�����ͬ�ĵ��Ӳ�ṹ��A��B��D��E�����γɹ��ۻ����A��B�γɵĻ�������ˮ�гʼ��ԣ�C��E�γɵĻ�������ˮ�г����ԡ�
�ش��������⣺
��1������Ԫ���У�ԭ�Ӱ뾶������ ���ǽ�������ǿ���� (��Ԫ�ط���)��
��2����A��B��D��E���γɵĹ����ͻ������У����ȶ��������� ���û�ѧʽ��ʾ����
��3��A��E�γɵĻ�������A��B�γɵĻ����ﷴӦ������Ļ�ѧʽΪ ,���д��ڵĻ�ѧ������Ϊ ��
��4������D�ڳ���ĵ���E��ȼ�գ���Ӧ�Ļ�ѧ����ʽΪ ��
��5������E��ˮ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijԪ�صĺ˵�����ǵ��Ӳ�����5��,����������������������3��,��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com