
��12�֣�a��b��c��dΪԭ��������������Ķ�����Ԫ�أ�b��c��dͬ���ڣ���a��b��c��d����Ԫ���γɵij�����ʽ��A����ͼ��ʾ��ת����ϵ��ͼ��ÿ����ĸ��ʾһ�ֵ��ʻ����
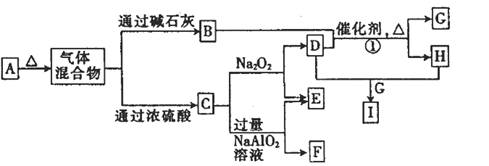
��1������C�ĽṹʽΪ________________��
��2��д���������ʵĻ�ѧʽ��F________��I________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ: ______________________��
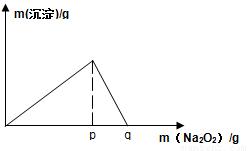
��4����F��һ���������ϣ�����ǡ�÷�Ӧ�õ���ҺM����M�м���Na2O2ʱ������Na2O2���������������������������ͼ��ʾ��ϵ����p��ʱ����������ų���������ʵ���֮��Ϊ ���ӿ�ʼ��Na2O2������q������������У��ܷ�Ӧ�����ӷ���ʽΪ ��
��4����A��E��ɵĹ�������X g ����ˮ�����Һ����������������I��ϡ��Һ����ü���I��Һ�����������C���������״�������±���ʾ����I��Һ�����ʵ���Ũ��Ϊ_____��
|
I ��Һ�������mL�� |
4 |
8 |
15 |
20 |
50 |
120 |
150 |
|
|
C�������mL�� |
0 |
0 |
112 |
224 |
896 |
2240 |
2240 |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| c2(SO3) |
| c2(SO2)?c(O2) |
| c2(SO3) |
| c2(SO2)?c(O2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | A | B | C | D |
| ���ʻ�ṹ��Ϣ | ��ҵ��ͨ������Һ̬��������䵥�ʣ���������ȼ | ��̬�⻯���Լ��� | +3�������ӵĺ�������Ų�����ԭ����ͬ | ��������ԭ�Ӱ뾶��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com