
��һ�������������̼���ơ������ơ�����ͭ���Ȼ��ơ��Ȼ��Ƶ�������ɡ�Ϊ�������ǣ���������ʵ�飺
(1)��������������ˮ����������ɫ��Һ��
(2)�ڴ���Һ�е����Ȼ�����Һ�а�ɫ�������ɣ�
(3)���ˣ�Ȼ���ڴ˰�ɫ�����м�������ϡ���ᣬ�������ȫ����ʧ��
�ɴ��ƶϣ����������п϶���________���϶�û��__________________________�����ܻ���__________�����Ҫ��һ��ȷ�������е������Ƿ���ڣ��ɲ��õļ��鷽����________________________________________________________________________
________________________________________________________________________��
(1)̼���ơ������ơ�����ͭ���Ȼ��ơ��Ȼ��ơ����������ữ��AgNO3��Һ�����а�ɫ�������ɣ�����NaCl������
����������֪����(1)�Ƴ��϶�������ɫCu2����Na2CO3��CaCl2ֻ������һ�֣�������(2)�Ƴ�Na2CO3��Na2SO4���ٺ���һ�֣�������(3)�Ƴ�ֻ��Na2CO3����Na2SO4�����(1)�Ľ��ۿɵ���CaCl2��
��Ҫȷ���Ƿ���NaCl��Ӧ����Cl���Ĵ��ڣ�ȡ����ԭ��Һ���Թ��У����������ữ��AgNO3��Һ�����а�ɫ�������ɣ�����NaCl��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л�������Ľṹ�����ʵ�������ȷ����(����)
A��������֬����ʹ����KMnO4��Һ��ɫ
B�������Cl2�ķ�Ӧ����ϩ��B r2�ķ�Ӧ����ͬһ���͵ķ�Ӧ
r2�ķ�Ӧ����ͬһ���͵ķ�Ӧ
C�����ǡ���ѿ�ǵķ���ʽ��ΪC12H22O11������Ϊͬ���칹��
D���Ҵ������������NaOH��Һ��Ӧ����Ϊ�����о����й����š���OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na��Al��Fe��Cu��Cl��Si��N��S����ѧ��ѧ����Ҫ��Ԫ�أ�������������Ԫ�ؼ��仯��������⣺
��1��д����ҵ�̵��ϳɰ��Ļ�ѧ����ʽ____________________________________��
��2����Na2Fe5Si8O22(OH)2 д�����������ʽΪ_________________________________��
��3��������Ϊ���ᣬ��H2S����ͨ�뵽FeCl3��Һ����Һ���ֵ���ɫ�ij�����д���÷�Ӧ�����ӷ���ʽ____________________________��
��4��д����Al��MnO2�����·�Ӧ��Mn�Ļ�ѧ����ʽ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ڽ���Ԫ�ؼס�����Ԫ�����ڱ��е����λ�����ұ���ʾ��
 �����ж���ȷ����( )
�����ж���ȷ����( )
A��ԭ�Ӱ뾶����>��>��
B�����ʵĻ�ԭ�ԣ���>��>��
C���ס��ҡ������������Ϊ���ۻ�����
D���ҡ�������������������Ӧ��ˮ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ֧25 mL����ʽ�ζ�����ʢ��0.1 mol·L��1 HCl��Һ����Һ��ǡ����5 mL�Ŀ̶ȴ������ѵζ����е���Һȫ�������ձ��У�Ȼ����0.1 mol·L��1 NaOH��Һ�����кͣ�������NaOH��Һ�����(����)
A������20 mL B����20 mL C������20 mL D������5 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼװ���У�ͨ���ɹ۲쵽Cu���ܽ⣬������˵���в���ȷ����(����)
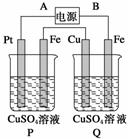
A��ֱ����Դ�У�A������
B��������CuSO4��ҺŨ�Ⱦ����ı�
C���������������������ͬ
D��P������Һ��������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C�����ձ��зֱ�ʢ����ͬŨ�ȵ�ϡ���ᣬ��ͼ��ʾ��
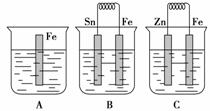
(1)A�з�Ӧ�����ӷ���ʽΪ_____________________________________________��
(2)B��Sn���ĵ缫��ӦʽΪ__________________________________________��
Sn��������Һ��pH________(���������С�����䡱)��
(3)C�б���ʴ�Ľ�����________���ܷ�Ӧ�����ӷ���ʽΪ________________________________���Ƚ�A��B��C��������ʴ�����ʣ��ɿ쵽����˳����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H2(g)��C2H4(g)��C2H5OH(l)��ȼ ���ȷֱ��ǣ�285.8 kJ·mol��1����1 411.0 kJ·mol��1�ͣ�1 366.8 kJ·mol��1������C2H4(g)��H2O(l)��Ӧ����C2H5OH(l)�Ħ�HΪ����
���ȷֱ��ǣ�285.8 kJ·mol��1����1 411.0 kJ·mol��1�ͣ�1 366.8 kJ·mol��1������C2H4(g)��H2O(l)��Ӧ����C2H5OH(l)�Ħ�HΪ����
A����44.2 kJ·mol��1������������B����44.2 kJ·mol��1
C����330 kJ·mol��1 D����330 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��amolС�մ��bmol������������ij����ɱ���ܱ������г�ּ��ȣ���Ӧ���������ڵ�����Ϊ1mol������˵��һ����ȷ����
A��b=2 B��������һ��û�в����CO2��ˮ����
C��a:b��1 D����Ӧ��ת�Ƶĵ�����һ��Ϊ2NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com