
(16��)�ȼҵ��������Ļ�ѧ��ҵ֮һ�����IJ�Ʒ��Ӧ���ڻ�ѧ��ҵ�����⣬���㷺Ӧ�����Ṥҵ����֯��ҵ��ұ��ҵ��ʯ�ͻ�ѧ��ҵ�Լ�������ҵ��
����ǰ������Ĥ������ʳ��ˮʱ��Cl2����NaOH�Ӵ��������п϶���NaClO���Ӷ�Ӱ���Ʒ�Ĵ��ȡ��ܰ�����һ���̵��ܷ�Ӧ����ʽΪ
���ִ��ȼҵ����ø�Ĥ�����е�⣬���ø�Ĥ�����۷ָ�����������������������������ų����� �����ʱ�����������������PH��2��3���û�ѧƽ���ƶ�ԭ��������������ã�
��3�� �ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30�����ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����
 �ٷ����Ƚ�ͼ���������c����������С��a%
(�����������������) b%, ������
�ٷ����Ƚ�ͼ���������c����������С��a%
(�����������������) b%, ������
��������Ƶ���Ҫ�ڣ��磩����֮������ ������дһ����
�� ������ԱΪ���ٻ������糧�Ի�������Ⱦ�����������û������糧���ȼ���оͽ����ϡ������жϸ÷����Ƿ���У� ������л��У���������
��1��NaCl+H2O NaClO+H2��
NaClO+H2��
��2��H2��NaOH�� Cl2��ˮ��Ӧ��Cl2+ H2O= HClO+HCl������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2�ݳ�
��3���٣�����B���Ҳ�Ϊȼ�ϵ����������������ԭ����OH-��ͬʱNa+ͨ������Ĥ�Ƶ��ò࣬����NaOH��ҺŨ������
��ȼ�ϵ�ؿ��Բ���������ĵĵ��ܣ���ȼ�ϵ������˲�����Һ��Ũ�ȣ������ܺĵ����������𰸾��ɣ�
��4�����У������糧�����ķ�����SO2�����ȼ������Cl2��NaOH��Ӧ���ȼ�����SO2���ŷţ��ֿɲ������ֻ�ѧԭ�ϣ�ͬʱ�ͽ����ϻ��ܼ��ٵ��������е���ģ����������𰸾��ɣ�
����������1���������Ǵ������ƺ������������ܷ�ӦʽΪNaCl+H2O NaClO+H2����
NaClO+H2����
��2�����Ե缫�缫�Ȼ�����Һ�������������ӷŵ磬�Ӷ��ƻ�������Χˮ�ĵ���ƽ�⣬�Ӷ�������Һ�Լ��ԣ����������������������������ơ������������ӷŵ���������������Cl2��ˮ��Ӧ��Cl2+ H2O= HClO+HCl������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2�ݳ���
��2���ٸ���װ��ͼ��֪��B���Ҳ�ͨ���������������������������ԭ����OH-��ͬʱNa+ͨ������Ĥ�Ƶ��ò࣬����NaOH��ҺŨ������
��������ƿ���ͨ��ȼ�ϵ��������������ĵĵ��ܡ�
��4�����ڷ��糧�����ķ�����SO2�����ȼ������Cl2��NaOH��Ӧ���ȼ�����SO2���ŷţ��ֿɲ������ֻ�ѧԭ�ϣ�ͬʱ�ͽ����ϻ��ܼ��ٵ��������е���ģ������ǿ��Եġ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��
��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
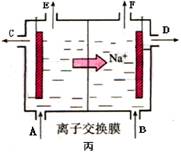
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������ʦ���и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ������
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��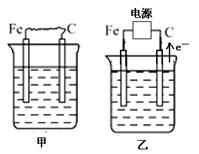

��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ܿ��ص������У�߶���ѧ�ڵ�һ��������ѧ������������ ���ͣ������
(16��)�ȼҵ��������Ļ�ѧ��ҵ֮һ�����IJ�Ʒ��Ӧ���ڻ�ѧ��ҵ�����⣬���㷺Ӧ�����Ṥҵ����֯��ҵ��ұ��ҵ��ʯ�ͻ�ѧ��ҵ�Լ�������ҵ��
����ǰ������Ĥ������ʳ��ˮʱ��Cl2����NaOH�Ӵ��������п϶���NaClO���Ӷ�Ӱ���Ʒ�Ĵ��ȡ��ܰ�����һ���̵��ܷ�Ӧ����ʽΪ
���ִ��ȼҵ����ø�Ĥ�����е�⣬���ø�Ĥ�����۷ָ�����������������������������ų����� �����ʱ�����������������PH��2��3���û�ѧƽ���ƶ�ԭ��������������ã�
��3���ȼҵ�Ǹߺ��ܲ�ҵ��һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խڣ��磩��30�����ϡ������ֹ�������У�������ϵĴ�����ת����ϵ����ͼ��ʾ�����еĵ缫δ��������õ�����Ĥ��ֻ����������ͨ����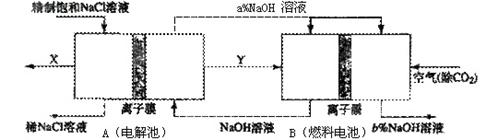 �ٷ����Ƚ�ͼ���������c����������С��a% (�����������������) b%, ������
�ٷ����Ƚ�ͼ���������c����������С��a% (�����������������) b%, ������
��������Ƶ���Ҫ�ڣ��磩����֮������ ������дһ����
�ȿ�����ԱΪ���ٻ������糧�Ի�������Ⱦ�����������û������糧���ȼ���оͽ����ϡ������жϸ÷����Ƿ���У� ������л��У���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ������
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��
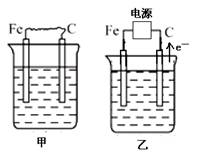
��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com