
| ���� ����ʽ��HCl ��Է���������36.5 �ܶȣ�1.19g/mL HCl������������36.5% |
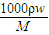 ����Ũ������HCl�����ʵ���Ũ�ȣ�
����Ũ������HCl�����ʵ���Ũ�ȣ�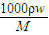 =
=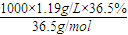 =11.9mol/L���ʴ�Ϊ��11.9mol/L��
=11.9mol/L���ʴ�Ϊ��11.9mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� ����ʽ��HCl ��Է���������36.5 �ܶȣ�1.19g/mL HCl������������36.5%��1����Ũ������HCl�����ʵ���Ũ��Ϊ 11.9mol/L 11.9mol/L ����2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯���� BD BD �����ţ���A����Һ��HCl�����ʵ���B����Һ��Ũ��C����Һ��Cl-����ĿD����Һ���ܶ� ��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.400mol/L��ϡ���ᣮ�ø�ѧ����Ҫ��ȡ 16.8 16.8 mL��С�������һλ������Ũ����������ƣ�������a��10mL��Ͳ��b��25mL��Ͳ��c���ձ���d��������ƽ��e.500mL����ƿ��f����ͷ�ιܣ�g�����������������ѡȡ��Ҫ������������������һ��ʹ���Ⱥ�˳������ bcgef��bfcge bcgef��bfcge �����ţ����������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����ƫ�ߡ�����ƫ�͡�����Ӱ�족��������ʱ���ӹ۲� ƫ�� ƫ�� �����ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮƫ�� ƫ�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��A�ࣩ�Իش��������⣺ ��1����֪24��A��40��Bǡ����ȫ��Ӧ����0.4molC��32��D����C��Ħ������Ϊ 80g/mol 80g/mol ����2����1molNa��lmol Mg�ֱ�Ͷ�뵽�����������У��ֱ�õ���Һa��b������Һa��b��������С��ϵΪma = = mb����3����ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
|