
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮���ת����ϵ����ͼ��ʾ�����ֲ�����ȥ����

��1����AΪ���ʣ����A���ʵ�Ԫ������Ȼ�����γɻ�������������Ԫ�ء�
��B���� ���ӣ�����ԡ��Ǽ��ԡ�����B����������ԭ���Ƿ�����8���ӽṹ�� (��ǡ���)��
����50 mL 4 mol/L��NaOH��Һ��ͨ��1.12 L B����״��������Ӧ����Һ�е�����Ϊ_______���ѧʽ�������ʵ����ֱ�Ϊ_____________��
��2����AΪ���������е�Ԫ�����γɵ��Ȼ��
��д��A��Һ��B�����ӷ���ʽ ��
��д��A��B����Һ�з�Ӧ�����ӷ���ʽ ��
��3����AΪ�����Ľ�������E���䡢Ũ��Һ���жۻ�������֪��XΪ���зǼ��Լ������ӻ������1 mol X����38 mol���ӣ���D��Һ�м�����D�����ʵ����Ĺ���X���÷�Ӧ�����ӷ���ʽΪ ��
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ�������
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
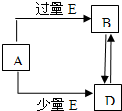 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ�������
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
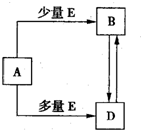 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
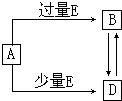 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com