
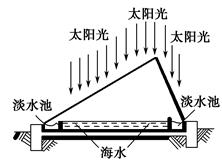
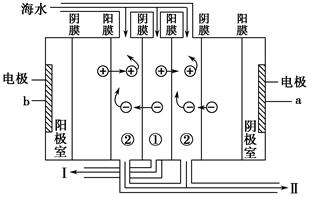
 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú��ʯ�ͺ���Ȼ��������̼��ȼ�� |
| B����չ̫���ܾ��������ڼ�������ЧӦ |
| C��̫���ܵ�ؿɽ�̫����ת��Ϊ���� |
| D��Ŀǰ�о����˵����ʡ����硱������̫�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢܢݢۢ� | B���ۢڢܢ٢� |
| C���ۢܢڢݢ� | D���ڢܢۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ������ˮ���ܽ��CO2һ���� |
| B����ˮ���ܽ��CO2�϶� |
| C����ˮ�е�c��HCO3-���ϴ� |
| D����ˮ�е�c��CO32-���ϴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
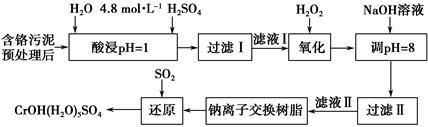
| ������ | Fe3�� | Mg2�� | Al3�� | Cr3�� |
| ��ʼ����ʱ��pH | 2.7 | �� | �� | �� |
| ������ȫʱ��pH | 3.7 | 11.1 | 8 | 9(>9�ܽ�) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
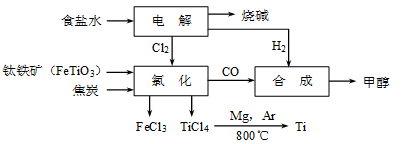
| | TiCl4 | Mg | MgCl2 | Ti |
| �۵�/�� | ��25.0 | 648.8 | 714 | 1667 |
| �е�/�� | 136.4 | 1090 | 1412 | 3287 |
 CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol��
CH3OH(g)�����������������������ʵ��κ���ʧ��������ҵ����ÿ�ϳ�6mol�״�����������ⲹ��H2 mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���л�����һ������̼��������Ԫ�� |
| B��һ�������£�ʹ�ô�������߷�Ӧ��ƽ��ת���� |
| C�����л�����С���ϡ����Բ���ѭԭ���غ㶨�� |
| D�����л�����С��ü�����Ҫ�ƻ��ɵĻ�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
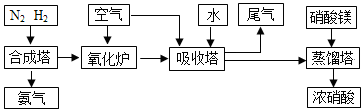
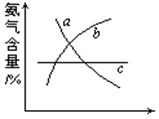
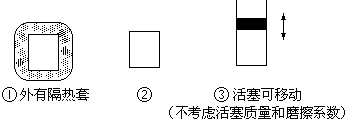
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com