
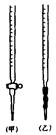
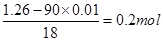 ���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2
���ʾ�����n(H2C2O4)��n(H2O)=1��2����x=2

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
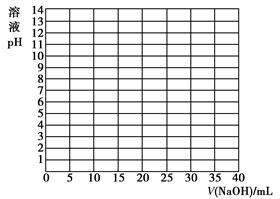
| V/(NaOH)/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 | 40.00 |
| ��ҺpH | 2.87 | 4.74 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 | 12.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����������� | ���͡����� |
| A�� | ͭƬ����Ũ�����У������Ա仯 | ͭ�����Ũ�����лᷢ���ۻ� |
| B�� | ��ij�Ȼ�����Һ�еμӰ�ˮ��������ɫ���� | ���Ȼ�����AlCl3 |
| C�� | ��10mlijpH=3��HA��Һ��ˮϡ�͵�100ml��������ҺpH=3.8 | HA������ |
| D�� | ��MgCl2��Һ�еμ�NaOH��Һ������pH=9ʱ����ʼ���ֳ���[��֪Mg(OH)2��Ksp=5.6��10-12] | ԭ��Һ�� c(Mg2+)=5.6��10-2mol��L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��ijƷ��������Ħ�����ɷּ��京����������̽����
��ijƷ��������Ħ�����ɷּ��京����������̽���� __________________________________________��
__________________________________________��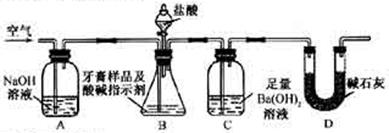
 ___��
___�� ��Ƶ���������Ϊ__________��
��Ƶ���������Ϊ__________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
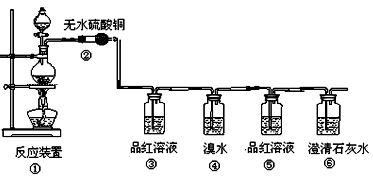
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʵ���� | �������� | �� Һ | ʵ��Ŀ�� |
| �� | ���Ͻ� | CO2�� H2O | ��̽����ͬ��Һ�Խ������ϵĸ�ʴ���ʣ� ��̽����ͬ�������ϵĸ�ʴ���ʣ� |
| �� | | | |
| �� | | |
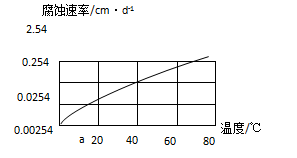
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
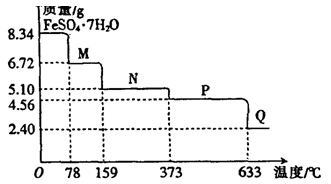
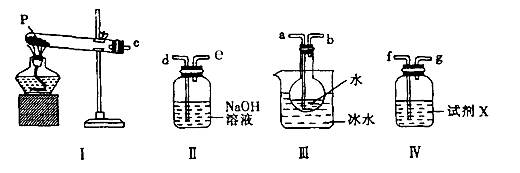
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
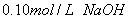 ��Һ��������һ�ݷ��ڿ�����һ��ʱ�����Һ��pH ���������С�����䡱����ԭ���� ������֪Ũ�ȵ������к�����������Һ�����кͷ��ڿ�����һ��ʱ�����Ƿ���Һ������������Ϊ
��Һ��������һ�ݷ��ڿ�����һ��ʱ�����Һ��pH ���������С�����䡱����ԭ���� ������֪Ũ�ȵ������к�����������Һ�����кͷ��ڿ�����һ��ʱ�����Ƿ���Һ������������Ϊ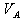 ���к���һ����Һ������������Ϊ
���к���һ����Һ������������Ϊ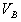 ����1���Լ���Ϊָʾ��
����1���Լ���Ϊָʾ��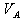 ��
��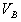 �Ĺ�ϵ�� ����2���Է�̪Ϊָʾ��ʱ��
�Ĺ�ϵ�� ����2���Է�̪Ϊָʾ��ʱ��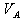 ��
��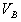 �Ĺ�ϵ�� ��
�Ĺ�ϵ�� ��| A����250mL������ƿ�ж������250mL�ռ���Һ |
| B������Һ����ȡ25mL�ռ���Һ����ƿ�в��μ���ָʾ������ |
| C������ƽ��ȷ��ȡ�ռ���ƷWg�����ձ���������ˮ�ܽ� |
| D�������ʵ���Ũ��ΪC�ı�������Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ����ΪV1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com