
| NaOHČÜŅŗ |
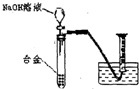 £¬
£¬ £®
£®


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ĒāŃõ»ÆÄĘČÜŅŗ |
| ŃĪĖį |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| NaOHČÜŅŗ |
| ŃĪĖį |

 ĪŹĢāĢÖĀŪ£ŗ
ĪŹĢāĢÖĀŪ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĻŗ£ŹŠĪāäĮ֊ѧø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
ĀĮĆ¾ŗĻ½šŅŃ³ÉĪŖĀÖ“¬ÖĘŌģ”¢»Æ¹¤Éś²śµČŠŠŅµµÄÖŲŅŖ²ÄĮĻ”£ŃŠ¾æŠŌѧĻ°Š”×éµÄČżĪ»Ķ¬Ń§£¬ĪŖ²ā¶Øijŗ¬Ć¾3£„Ņ»5£„µÄĀĮĆ¾ŗĻ½š(²»ŗ¬ĘäĖüŌŖĖŲ)ÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬Éč¼ĘĻĀĮŠČżÖÖ²»Ķ¬ŹµŃé·½°ø½ųŠŠĢ½¾æ”£ĢīŠ“ĻĀĮŠæÕ°×”£
”¾Ģ½¾æŅ»”æŹµŃé·½°ø£ŗĀĮĆ¾ŗĻ½š ²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣
²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣
ĪŹ Ģā£ŗŹµŃéÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ŹµŃé²½Öč£ŗ
(1)³ĘČ”5.4gĀĮĆ¾ŗĻ½š·Ūĩѳʷ£¬Ķ¶ČėVmL 2”¢0mol”¤L-1NaOHČÜŅŗÖŠ£¬³ä·Ö·“Ó¦”£NaOHČÜŅŗµÄĢå»żV”Ż
(2)¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæ¹ĢĢ唣øĆ²½ÖčÖŠČōĪ“Ļ“µÓ¹ĢĢ壬²āµĆĆ¾µÄÖŹĮæ·ÖŹż½« (Ģī”°Ę«øß”±»ņ”°Ę«µĶ”±)”£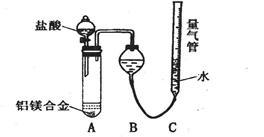
”¾Ģ½¾æ¶ž”æŹµŃé·½°ø£ŗĀĮĆ¾ŗĻ½š ²ā¶ØÉś³ÉĘųĢåµÄĢå»żŹµŃé×°ÖĆ”£
²ā¶ØÉś³ÉĘųĢåµÄĢå»żŹµŃé×°ÖĆ”£
ĪŹĢāĢÖĀŪ£ŗ
(1)ijĶ¬Ń§Ģį³öøĆŹµŃé×°ÖĆ²»¹»ĶźÉĘ£¬Ó¦ŌŚA”¢BÖ®¼äĢķ¼ÓŅ»øöøÉŌļ”¢³żĖįĪķµÄ×°ÖĆ”£ÄćµÄŅā¼ūŹĒ£ŗ (Ģī”°ŠčŅŖ”±»ņ”°²»ŠčŅŖ”±)”£
(2)ĪŖŹ¹²ā¶Ø½į¹ū¾”æÉÄܾ«Č·£¬ŹµŃé֊ӦעŅāµÄĪŹĢāŹĒ(Š“³öĮ½µć)£ŗ
¢Ł
¢Ś 
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģø£½ØŹ”øßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ĀĮĆ¾ŗĻ½šŅŃ³ÉĪŖĀÖ“¬ÖĘŌģ”¢»Æ¹¤Éś²śµČŠŠŅµµÄÖŲŅŖ²ÄĮĻ”£ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§£¬ĪŖ²ā¶Øij²»Ķ¬Ę·ÅĘĀĮĆ¾ŗĻ½š£Ø²»ŗ¬ĘäĖüŌŖĖŲ£©ÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬Éč¼ĘĻĀĮŠĮ½ÖÖ²»Ķ¬ŹµŃé·½°ø½ųŠŠĢ½¾æ”£ĢīŠ“ĻĀĮŠæÕ°×”£
[Ģ½¾æŅ»] ŹµŃé·½°ø£ŗĀĮĆ¾ŗĻ½š ²ā¶ØÉś³ÉĘųĢåµÄĢå»ż
²ā¶ØÉś³ÉĘųĢåµÄĢå»ż
ŹµŃé×°ÖĆČēĻĀĶ¼£¬ĪŹĢāĢÖĀŪ£ŗ

£Ø1£©·“Ó¦Ķź±Ļ,Ćæ¼äøō1·ÖÖÓ¶ĮČ”ĘųĢåĢå»ż£¬ĘųĢåĢå»żÖš½„¼õŠ”£¬Ö±ÖĮ²»±ä”£ĘųĢåĢå»ż¼õŠ”µÄŌŅņŹĒ
£ØŅĒĘ÷ŗĶŹµŃé²Ł×÷µÄÓ°ĻģŅņĖŲ³żĶā£©”£
£Ø2£©ĪŖŹ¹²ā¶Ø½į¹ū¾”æÉÄܾ«Č·£¬ŹµŃé֊ӦעŅāµÄĪŹĢā³żĮĖ¼ģ²é×°ÖƵÄĘųĆÜŠŌ”¢¼ÓČė×ćĮæŃĪĖįŹ¹ŗĻ½šĶźČ«ČܽāŗĶ°“£Ø1£©²Ł×÷Ķā£¬ĒėŌŁŠ“³öĮ½µć£ŗ
¢Ł
¢Ś
£Ø3£©Čē¹ūÓĆ·ÖĪöĢģĘ½×¼Č·³ĘČ”0.51gĆ¾ĀĮŗĻ½ųŠŠŹµŃ飬²āµĆÉś³ÉĘųĢåĢå»żĪŖ560 mL£ØŅŃÕŪĖć³É±źæöĻĀĢå»ż£©£¬Ēė¼ĘĖćŗĻ½šÖŠĆ¾µÄÖŹĮæ·ÖŹż”££ØĒėŠ“³ö¼ĘĖć¹ż³Ģ£©___________ ____________________________________________________________________________”£
[Ģ½¾æ¶ž] ŹµŃé·½°ø£ŗ³ĘĮæBgĮķŅ»Ę·ÅĘĀĮĆ¾ŗĻ½š·ŪÄ©£®·ÅŌŚČēĻĀĶ¼ĖłŹ¾×°ÖƵĶčŠŌµēČČ°åÉĻ£¬ĶصēŹ¹Ęä³ä·Ö×ĘÉÕ”£
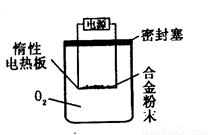
ĪŹĢāĢÖĀŪ£ŗ
£Ø1£©Óū¼ĘĖćMgµÄÖŹĮæ·ÖŹż£¬øĆŹµŃéÖŠ»¹Šč²ā¶ØµÄŹż¾ŻŹĒ ”£
£Ø2£©ÓƱ¾·½°ø½ųŠŠŹµŃ鏱£¬×°ÖĆÖŠÖĮÉŁŅŖ³äČėO2µÄĪļÖŹµÄĮæ mol£ØÓĆŗ¬BµÄ×ī¼ņŹ½±ķŹ¾£©”£
[ŹµŃéĶŲÕ¹] ŃŠ¾æŠ”×é¶ŌijĪŽÉ«ĶøĆ÷µÄČÜŅŗ½ųŠŠŹµŃ飬·¢ĻÖøĆČÜŅŗøśĀĮ·“Ó¦Ź±·Å³öH2£¬ŹŌÅŠ¶ĻĻĀĮŠĄė×Ó£ŗMg2+”¢Cu2+”¢Ba2+”¢H+”¢Ag+”¢SO42£”¢HCO3£”¢OH£”¢NO3££¬ŌŚĻĀĮŠĮ½ÖÖĒéæöĻĀ,æÉÄÜ“ęŌŚÓŚ“ĖČÜŅŗÖŠµÄŹĒ£ŗ
¢Łµ±ÓėĀĮ·“Ó¦ŗóÉś³ÉAl3+Ź±£¬ŌČÜŅŗæÉÄÜ“óĮæ“ęŌŚµÄĄė×Ó ”£
¢Śµ±ÓėĀĮ·“Ó¦ŗóÉś³É[Al(OH)4]£Ź±£¬ŌČÜŅŗæÉÄÜ“óĮæ“ęŌŚµÄĄė×ÓŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com