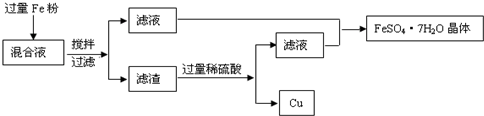
�������ɱ������ݿ�֪��ʵ��٢ڶ��н���ʣ�࣬����Һ�в����ܺ�������������Һ�н�������Ϊ+2�ۣ���ʵ��ٵĻ����ϼ���100mL���ᣬ�μӷ�Ӧ�Ľ���������Ϊ18g-9.6g=8.4g��
����NO�����Ϊ4480mL-2240mL=2240mL��NO���ʵ���Ϊ0.1mol��
���ݵ���ת���غ��֪���μӷ�Ӧ���������ʵ���Ϊ
=0.15mol��
�μӷ�Ӧ������ƽ��Ħ������Ϊ
=56g/mol��
�ʸù���ֻ��Fe�μӷ�Ӧ����ʵ���ֻ��Fe���뷴Ӧ��
����NO�������Ϸ���ʽ������������ʵ������μӷ�ӦFe�����ʵ�����
����c=
������������ʵ���Ũ�ȣ�����m=nM������вμӷ�ӦFe��������
��ʵ��ڵĻ����ϼ���100mL���ᣬ�μӷ�Ӧ�Ľ���������Ϊ9.6g������NO�����Ϊ6720mL-4480mL=2240mL��NO���ʵ���Ϊ0.1mol��
���ù���ֻ��Cu�μӷ�Ӧ�����ݵ���ת���غ㣬
��Cu�����ʵ���=
=0.15mol��Cu������=0.15mol��64g/mol=9.6g�����ڲμӷ�Ӧ������������
�ʸù���ֻ��Cu��Ӧ����Cuǡ����ȫ��Ӧ��
�ʼ���200mL����ʱ��Feǡ�÷�Ӧ����������������ʵ��۵Ļ������ټ���100mL���ᣬΪ��������Һ���������ӷ�Ӧ����NO��
�����������ӵ����ʵ��������õ���ת���غ����ý�����NO�����ʵ������ٸ���V=nV
m����ý�����NO���������������V��ֵ��
�����ܽ�Ľ���Ϊ�����ܽ��Fe���ڢٵĻ����ϼ���100mL�����ܽ�Ľ���������֮�ͣ�
ÿ�ݽ��������Ϊ�����ܽ��Fe��ʵ���ʣ��Ľ���������֮�ͣ�
����⣺�ɱ������ݿ�֪��ʵ��٢ڶ��н���ʣ�࣬����Һ�в����ܺ�������������Һ�н�������Ϊ+2�ۣ���ʵ��ٵĻ����ϼ���100mL���ᣬ�μӷ�Ӧ�Ľ���������Ϊ18g-9.6g=8.4g��
����NO�����Ϊ4480mL-2240mL=2240mL��NO���ʵ���Ϊ0.1mol��
���ݵ���ת���غ��֪���μӷ�Ӧ���������ʵ���Ϊ
=0.15mol��
�μӷ�Ӧ������ƽ��Ħ������Ϊ
=56g/mol��
�ʸù���ֻ��Fe�μӷ�Ӧ����ʵ���ֻ��Fe���뷴Ӧ��
��ʵ��ڵĻ����ϼ���100mL���ᣬ�μӷ�Ӧ�Ľ���������Ϊ9.6g������NO�����Ϊ6720mL-4480mL=2240mL��NO���ʵ���Ϊ0.1mol��
���ù���ֻ��Cu�μӷ�Ӧ�����ݵ���ת���غ㣬
��Cu�����ʵ���=
=0.15mol��Cu������=0.15mol��64g/mol=9.6g�����ڲμӷ�Ӧ������������
�ʸù���ֻ��Cu��Ӧ����Cuǡ����ȫ��Ӧ��
�ʼ���200mL����ʱ��Feǡ�÷�Ӧ����������������ʵ��۵Ļ������ټ���100mL���ᣬΪ��������Һ���������ӷ�Ӧ����NO��
��1��������������֪��ʵ��ٷ�����Ӧ3Fe+8HNO
3�T3Fe��NO
3��
2+2NO��+4H
2O������NO�����ʵ���Ϊ
=0.1mol��
���ݷ���ʽ��֪���μӷ�Ӧ����������ʵ���Ϊ0.1mol��4=0.4mol��
����������ʵ���Ũ��=
=4mol/L��
�ʴ�Ϊ��4��
��2��������������֪��ʵ��ٷ�����Ӧ3Fe+8HNO
3�T3Fe��NO
3��
2+2NO��+4H
2O������NO�����ʵ���Ϊ0.1mol��
���ݷ���ʽ��֪���μӷ�Ӧ��Fe�����ʵ���Ϊ0.1mol��
=0.15mol��
�ʲμӷ�ӦFe������=0.15mol��56g/mol=8.4g��
�ʴ�Ϊ��8.4��
��3���ɣ�2��֪�����ܽ���8.4gFe����ʵ��ٵĻ����ϼ���100mL���ᣬ�μӷ�Ӧ�Ľ���������Ϊ18g-9.6g=8.4g��
��ʵ��ڹ��ܽ��������Ϊ8.4g+8.4g=16.8g��
�ʴ�Ϊ��16.8g��
��4���ɣ�2��֪�����ܽ���8.4gFe����ÿ����Һ�к��н���������Ϊ8.4g+18g=26.4g��
�ʴ�Ϊ��26.4��
��5��������������֪��ʵ�����Fe������ǡ�÷�Ӧ������������������NO�����ʵ���=
=0.2mol��
��3Fe+8HNO
3�T3Fe��NO
3��
2+2NO��+4H
2O��֪����Һ���������ӵ����ʵ���Ϊ0.3mol����ʵ��۵Ļ������ټ���100mL���ᣬΪ��������Һ���������ӷ�Ӧ����NO��
���ݵ���ת���غ��֪������NO�����ʵ���=
=0.1mol��
������NO�����Ϊ0.1mol��22.4L/mol=2.24L=2240mL��
��V=6720+2240=8960��
�ʴ�Ϊ��8960��



 ��У����ϵ�д�
��У����ϵ�д�