
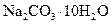 ��������
��������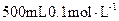 ��
��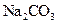 ��Һ����ش��������⡣
��Һ����ش��������⡣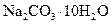 ����_______g��
����_______g��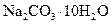 ������__________g��
������__________g��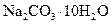 ������ʧȥ���ֽᾧˮ��____________
������ʧȥ���ֽᾧˮ��____________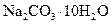 �����л�������
�����л�������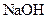 ���壬���������Ƶ�
���壬���������Ƶ�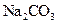 ��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱����
��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱���� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
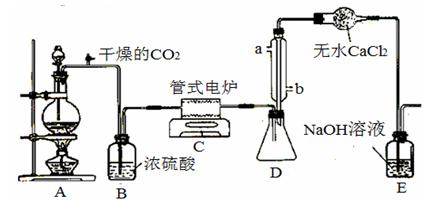
 ��CO32-��OH
��CO32-��OH ��SO
��SO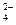 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩.
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ������������ˮ�⣩. L-1 H2SO4 b��0.01mol
L-1 H2SO4 b��0.01mol L-1 KMnO4 c��1mol
L-1 KMnO4 c��1mol L-1 BaCl2
L-1 BaCl2| ʵ�鲽�� |  Ԥ������ͽ��� Ԥ������ͽ��� |
����1��ȡ��������Һ���Թ��У��μ�3 mol L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��С� L-1 H2SO4����Һ�����ԣ�Ȼ��������Һ������A��B�Թ��С� | |
| ����2����A�Թ��еμ�1��2�� ������ţ��� | ����Һ �������1������ ���������2��3������ |
| ����3����B�Թ��еμ�1��2�� ������ţ��� | ����Һ �������3������ ����ϲ���2�������2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
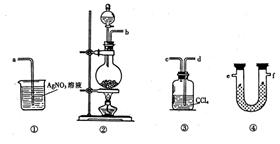
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
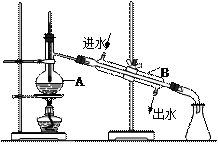
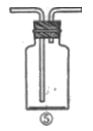
 ��B������������ ��
��B������������ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
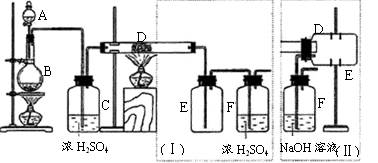
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
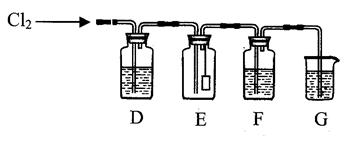
 ����ʽ ��
����ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ�����Ʊ�O2����H2O2��MnO2Ϊԭ�Ϸ����Ƶ� |
| B��������ᷢչʷ��Ҳ���������ұ��ʷ�����ڻ�ѧ�����������ý������������ȷ��ֲ��Ƶã����Ż�ѧ��չ�����ý��������Ƿ��ֲ��Ƶã��������ͭ��ͭ�������������������������ơ� |
| C����ҵ�Ʊ�þ���õ�������Ȼ�þ�ķ��� |
| D����ҵ���õ�ⱥ��ʳ��ˮ�ķ����Ʊ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com