
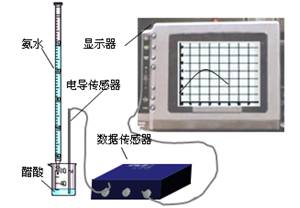
DISϵͳ����ѧ����Ϣϵͳ�����ɴ����������ݲɼ����ͼ������ɣ�ijѧϰС����DISϵͳ�ⶨʳ�ô��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㡣ʵ�鲽�����£�
|


��1���� �����������ƣ���ȡ10.00mLʳ�ð״ף��� �����������ƣ�����ˮϡ�ͺ�ת�Ƶ�100mL�� �����������ƣ��ж��ݣ�Ȼ��ϡ�ͺ����Һ�����Լ�ƿ�С�
��2����ȡ20.00 mL������Һ�����ձ��У����Ӻ�DISϵͳ�����ձ��еμ�Ũ��Ϊ0.1000mol?L-1�İ�ˮ���������Ļ����ʾ����Һ���������氱ˮ����仯�����ߣ�������ͼ��.
���õζ���ʢ��ˮǰ���ζ���Ҫ�� ��ϴ2�D3�飬��ϴ��Ŀ���� ��
�ڰ�ˮ����ᷴӦ�����ӷ���ʽ�� ��
��ʳ�ð״��д�������ʵ���Ũ���� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2009����������һ�и����꼶�ڶ����¿��������ۺϻ�ѧ���� ���ͣ�058
DISϵͳ����ѧ����Ϣϵͳ�����ɴ����������ݲɼ����ͼ������ɣ�ijѧϰС����DISϵͳ�ⶨʳ�ô��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㣮ʵ�鲽�����£�

(1)��________(����������)��ȡ10.00 mLʳ�ð״ף���________(����������)����ˮϡ�ͺ�ת�Ƶ�100 mL��________(����������)�ж��ݣ�Ȼ��ϡ�ͺ����Һ�����Լ�ƿ�У�
(2)��ȡ20.00 mL������Һ�����ձ��У����Ӻ�DISϵͳ�����ձ��еμ�Ũ��Ϊ0.1000 mol��L��1�İ�ˮ���������Ļ����ʾ����Һ���������氱ˮ����仯������(����ͼ)��

���õζ���ʢ��ˮǰ���ζ���Ҫ��________��ϴ2��3�飬��ϴ��Ŀ����________________��
�ڰ�ˮ����ᷴӦ�����ӷ���ʽ��_____________��
��ʳ�ð״��д�������ʵ���Ũ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��_________(����������)��ȡ10.00 mLʳ�ð״ף���___________(����������)����ˮϡ�ͺ�ת�Ƶ�100 mL��__________ (����������)�ж��ݣ�Ȼ��ϡ�ͺ����Һ�����Լ�ƿ�С�
(2)��ȡ20.00 mL������Һ�����ձ��У����Ӻ�DISϵͳ�����ձ��еμ�Ũ��Ϊ0.100 0 mol��L-1�İ�ˮ���������Ļ����ʾ����Һ���������氱ˮ����仯������(����ͼ)��
���õζ���ʢ��ˮǰ���ζ���Ҫ��____________________________________��ϴ2��3�飬��ϴ��Ŀ����_____________________________________________��
�ڰ�ˮ����ᷴӦ�����ӷ���ʽ��____________________________________��
��ʳ�ð״��д�������ʵ���Ũ����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ÷���и߶�12���¿���ѧ�Ծ� ���ͣ�ʵ����
��8�֣�DISϵͳ�����ֻ���Ϣϵͳ�����ɴ����������ݲɼ����ͼ������ɣ�ij�о���ѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㡣ʵ�鲽�����£�
����_______________�����������ƣ���ȡ10.00mLʳ�ð״ף���_________�����������ƣ�����ˮϡ�ͺ�ת�Ƶ�100mL___________�����������ƣ��ж��ݣ�Ȼ��ϡ�ͺ����Һ�����Լ�ƿ�С�
����ȡ20.00mL������Һ�����ձ��У����Ӻ�DISϵͳ��������ͼ�������ձ��еμ�Ũ��Ϊ0.1000mol/L�İ�ˮ���������Ļ����ʾ����Һ���������氱ˮ����仯�����ߣ�������ͼ����
���õζ���ʢ��ˮǰ���ζ���Ҫ��_________��ϴ2��3�飬��ϴ��Ŀ����____________��
�ڰ�ˮ����ᷴӦ�����ӷ���ʽ��__________________________________________��
��ʳ�ð״��д�������ʵ���Ũ����_____________��
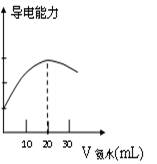
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(7��) DISϵͳ�����ֻ���Ϣϵͳ�����ɴ����������ݲɼ����ͼ������ɣ�ijѧϰС����DISϵͳ�ⶨʳ�ð״��д�������ʵ���Ũ�ȣ�����Һ�ĵ����������жϵζ��յ㡣ʵ�鲽�����£�
��1) ��_________________(���������ƣ���ȡ10. 00mL��ʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL________�����������ƣ��ж��ݣ�Ȼ��ϡ�ͺ����Һ�����Լ�ƿ�С�
(2)��ȡ20. 00 mL��������Һ�����ձ��У����Ӻ�DIS ϵͳ������ͼ�������ձ��еμ�Ũ��Ϊ0.1000mol��L -1�İ�ˮ���������Ļ����ʾ����Һ����������
��ˮ����仯�����ߣ���ͼ����
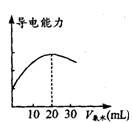
���õζ���ʢ��ˮǰ���ζ���Ҫ��__________��ϴ2��3��
�ڰ�ˮ����ᷴӦ�����ӷ���ʽ��___________________��
��ʳ�ð״��д�������ʵ���Ũ����__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com