
��16�֣�ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Ρ��ڷ�Ӧ�����Һ�У���μ���4mol��L��1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺
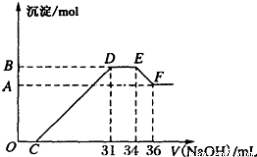
��1��DE�η�����Ӧ�����ӷ���ʽΪ��_____________________________________ ��
��2����д������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________ ��
��3��B���Ӧ�ij��������ʵ���Ϊ_______mol��C���Ӧ������������Һ�����Ϊ______mL��
��4��ԭ������Һ�����ʵ���Ũ��Ϊ_______mol/L��
(1) NH4++OH��=NH3?H2O
(2) 8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3=8Fe(NO3)3+3NH4NO3+9H2O
(3) 0.032�� 7 ��(4) 0.074��
��������
������������������֪����O��C�η�����Ӧ��H++OH-=H2O���������������������Һ��FeԪ������Fe3+���ڣ���C��D������Ӧ��Al3++3OH-=Al(OH)3��;Fe3++3OH-=Fe(OH)3��;(1)��D��E�η�����Ӧ��NH4++OH��=NH3?H2O����E��F�㷢����Ӧ��Al(OH)3+OH-=AlO2-+2H2O��(2)�������� Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽ��8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3=8Fe(NO3)3+3NH4NO3+9H2O����3���ܽ�Al(OH)3���ĵ�NaOH�������2ml,���γ�Al(OH)3�������ĵ�NaOH�������6ml, n(NH4+) =0.003ml��4mol/L=0.012mol,���ݵ����غ��֪n(e-)=0.012mol��8=0.096mol,����Al��Fe����+3�۵Ľ���������n(Al(OH)3)+ n(Fe(OH)3)= 0.096mol��3=0.032mol����B���Ӧ�ij��������ʵ���Ϊ0.032mol���ܽ�Al(OH)3����NaOH�����ʵ�����4mol/L��0.002L=0.008mol��ʹ���������γɳ������ĵ�OH-�����ʵ��������ת�Ƶ����ʵ�����ȣ�n(OH-)=0.096mol����ʹ���������γɳ������ĵ�NaOH������ǣ�V(NaOH)= 0.096mol��4mol/L=0.024L=24ml.����C���Ӧ������������Һ�����Ϊ31ml��24ml=7ml����4���������ﵽ���ֵʱ����Һ��NԪ�صĴ�����NaNO3��NH4NO3��n(HNO3)= 0.031L��4mol/L+ 2��0.012mol= 0.148mol������c(HNO3)=n��V=0.148mol��2L=0.074mol/L��
���㣺����ͼ���ڱ�ʾ���ʵķ�Ӧ�����е����ʱ仯����Ӧ�����֪ʶ��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ��������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����H++OH- �� H2O��ʾ���� ( )
A��Ba(OH)2��Һ��ϡH2SO4�ķ�Ӧ B��NaOH��Һ�����ᷴӦ
C��Cu(OH)2��ϡH2SO4�ķ�Ӧ D��NaOH��Һ��CO2�ķ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ����9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵ�Ӧ�ò��漰������ԭ��Ӧ���� �� ��
A����ϡ����ϴȥ�����Թ��ڱڵ�����
B�����ȵĴ�����Һ��ϴ����
C�������Ƶ�������ͭ����Һ�벡�˵���Һ��ϼ��ȣ������鲡���Ƿ�����
D�������ʶƼ��϶�ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭���ϲ����и���11�·��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���20 mL 0��2 mol/L H2A��Һ�еμ�0��2 mol/L NaOH��Һ���й��������ʵ����仯����ͼ(���Т����H2A�������HA���������A2��)������ͼʾ�жϣ�����˵����ȷ����
A��H2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A===H����HA����HA��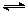 H����A2��
H����A2��
B����V(NaOH)��20 mLʱ����Һ�и�����Ũ�ȵĴ�С˳��Ϊ��c(Na��)>c(HA��)>c(H��)>c(A2��)>c(OH��)
C���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ��
D����V(NaOH)��30 mLʱ����Һ�д������¹�ϵ��2c(H��)��c(HA��)��2c(H2A)��c(A2��)��2c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭���ϲ����и���11�·��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
A���������м�������ϡ���2Fe��6H+��2Fe3����3H2��
B����AlCl3��Һ�еμ�Ũ��ˮ��������Al3+��4OH����AlO2����2H2O
C����NaIO3��Һ�м�������NaHSO3��Һ��IO3����3HSO3����I����3SO42����3H+
D����NH4HCO3��Һ�еμӹ���NaOH��Һ��NH4+��HCO3����2OH����CO32����NH3��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭�����Ƹ��и����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�谢���ӵ�����ΪNA������˵����ȷ���ǣ� ��
A����״����,VL��������������ΪV/22.4NA
B��1.5 mol NO2������H2O��Ӧ��ת�Ƶĵ�����ΪNA
C�����³�ѹ��,0.2mol��SiO2����������Ϊ0.2NA
D��31g����(P4)���3/2NA��P��P��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭�����Ƹ��и����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�������һ���ܴ���������ǣ� ��
A���ں��д�����Al(OH)4�ݣ�����Һ�У�NH4����Na����Cl����H��
B����ǿ����Һ�У�Na����K����CO32����NO3��
C����pH��12����Һ�У�NH4����Na����SO42����Cl��
D����c(H+)��0.1mol��L��1����Һ�У�K����I����Cl����NO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�γ��и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ѧģ�����о���ѧ���⣬��ֱ���ּ�ࡣ���н���������ģ����ȷ����
A������������ȼ�գ��Ƶ��������
B������Cl2��ȼ�գ������������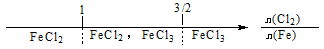
C��NH3��Cl2��Ӧ����Ӧ���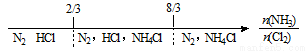
D��FeBr2��Һ��ͨ��Cl2����Ԫ�ش�����ʽ��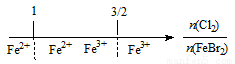
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��BaO2�����ܱ���������У���Ӧ2BaO2(s)  2BaO(s)+O2(g)�ﵽƽ�⣬�����¶Ȳ��䣬��С�����ݻ�����ϵ���´ﵽƽ��ʱ������˵����ȷ����
2BaO(s)+O2(g)�ﵽƽ�⣬�����¶Ȳ��䣬��С�����ݻ�����ϵ���´ﵽƽ��ʱ������˵����ȷ����
A��ƽ�ⳣ����С B��BaO������
C������Ũ������ D��BaO2������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com