
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���________���ڵ�________�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ________________________��
(2)�á�>����<����գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si____S | O2��____Na�� | NaCl____Si | H2SO4____HClO4 |
(3)CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ���ʼ��仯����Ӧ�÷�Χ�ܹ㡣���к��Ȼ��������ʵ��Ȼ�ͭ����(CuCl2��2H2O)��Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ����ͼ��������ᴿ��
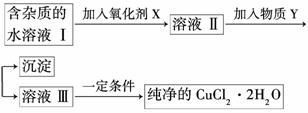
��֪Cu2����Fe3����Fe2�����������↑ʼ�����ͳ�����ȫʱ��pH���±���
| Fe3�� | Fe2�� | Cu2�� | |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |
��ش��������⣺
(1)������������Ŀ����__ ______________________��
______________________��
(2)���ʺ���������X����__________��
A��K2Cr2O7 B��NaClO
C��H2O2 D��KMnO4
(3)���������Y��__________��
(4)����������Y����ֱ���ü��ܲ��ܴﵽĿ�ģ�______(��ܡ����ܡ�)�����ܣ����ûش������ܣ��Խ���ԭ��
_________________________________________ _______________________________��
_______________________________��
(5)����ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O���壿________(��ܡ����ܡ�)�����ܣ����ûش������ܣ��ش����β�����____________________________________��
(6)������Һ���м���̼��ƣ�������������______________________________��
( 7)������Һ���м���þ�ۣ�������������____________���Խ���ԭ��____________________________________________________��
7)������Һ���м���þ�ۣ�������������____________���Խ���ԭ��____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧΪ̽��SO2��Ba2��ֻ���ڼ��������²����γ�BaSO3��������������·���������Ϊ���е���
A����SO2ͨ��Ba(OH)2��Һ�й۲��а�ɫ��������
B����SO2ͨ��Ba(NO3)2��Һ�й۲��а�ɫ��������
C����SO2�ֱ�ͨ��BaCl2��Һ��BaCl2��HCl�Ļ����Һ��Ba(OH)2��Һ�У��۲쵽ֻ��Ba(OH)2���а�ɫ��������
D����SO2ͨ��BaCl2��NH3�Ļ����Һ���а�ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�Ag2SO4��AgCl��AgI���ܶȻ���������Ϊ��Ksp(Ag2SO4)��7.7��10��5��Ksp(AgCl)��1.8��10��10��Ksp(AgI)��8.3��10��17�������й�˵���У��������
(����)
A�������£�Ag2SO4��A gCl��AgI��ˮ�е��ܽ��������μ���
gCl��AgI��ˮ�е��ܽ��������μ���
B����AgCl������Һ�м���NaI���壬��AgI��������
C��Ag2SO4��AgCl��AgI���ܶȻ�����֮�ȵ������DZ�����Һ�����ʵ���Ũ��֮��
D����Ag2SO4������Һ�м���Na2SO4������Ag2SO4��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ��ˮ�г�����һ������Cr2O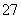 ��CrO
��CrO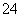 �����ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�
�����ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ
CrO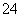
 Cr2O
Cr2O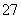
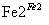 Cr3��
Cr3��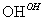 Cr(OH)3��
Cr(OH)3��
���еڢٲ�����ƽ�⣺
2CrO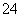 (��ɫ)��2H��
(��ɫ)��2H�� Cr2O
Cr2O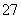 (��ɫ)��H2O
(��ɫ)��H2O
(1)��ƽ����ϵ�� pH �� 2������Һ��________ɫ��
(2)��˵���ڢٲ���Ӧ��ƽ��״̬����________��
a��Cr2O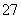 ��CrO
��CrO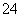 ��Ũ����ͬ
��Ũ����ͬ
b��2v(Cr2O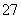 )��v(CrO
)��v(CrO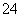 )
)
c����Һ����ɫ����
(3)�ڢڲ��У���ԭ 1 mol Cr2O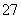 ���ӣ���Ҫ________mol��FeSO4��7H2O��
���ӣ���Ҫ________mol��FeSO4��7H2O��
(4)�� �۲����ɵ�Cr(OH)3 ����Һ�д������³����ܽ�ƽ�⣺
Cr(OH)3(s)  Cr3��(aq)��3OH��(aq)
Cr3��(aq)��3OH��(aq)
�����£�Cr(OH)3 ���ܶȻ�Ksp��c(Cr3��)��c3(OH��)��10��32��Ҫʹ c(Cr3��)����10��5mol/L����Һ��pHӦ����________��
���ܶȻ�Ksp��c(Cr3��)��c3(OH��)��10��32��Ҫʹ c(Cr3��)����10��5mol/L����Һ��pHӦ����________��
����2����ⷨ
�÷��� Fe ���缫��⺬Cr2O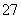 �����Է�ˮ�����ŵ��Ľ��У�������������Һ pH ���ߣ����� Cr(OH)3 ������
�����Է�ˮ�����ŵ��Ľ��У�������������Һ pH ���ߣ����� Cr(OH)3 ������
(5)��Fe���缫��ԭ��Ϊ_____________________ _______________________��
_______________________��
(6)������������Һ pH ���ߵ�ԭ����(�õ缫��Ӧ����)________����Һ��ͬʱ���ɵij�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a�ĺ����������������������ͬ��b�ļ۵��Ӳ���δ�ɶԵ�����3����c������������Ϊ���ڲ��������3����d��cͬ�壻e�������ֻ��1�����ӣ����������18�����ӡ��ش��������⣻
(1)b��c��d�е�һ������������________(��Ԫ�ط���)��e�ļ۲���ӹ��ʾ��ͼΪ________��
(2)a������Ԫ���γɵĶ�Ԫ���ۻ������У����ӳ������Σ��÷��ӵ�����ԭ�ӵ��ӻ���ʽΪ______�������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������________(�ѧʽ��д������)��
(3)��ЩԪ���γɵĺ������У����ӵ�����ԭ�ӵļ۲���Ӷ���Ϊ3������________������������ṹ������________��(�ѧʽ)
(4)e��c�γɵ�һ�����ӻ�����ľ���ṹ��ͼ(a)����e���ӵĵ��Ϊ________��
(5)��5��Ԫ���γɵ�һ��1��1�����ӻ������У������ӳ�������ṹ�������ӳ����������İ�����ṹ[��ͼ(b)��ʾ]��
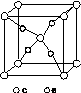 ������
������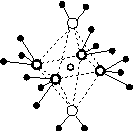
(a)����������������������(b)
�û������У�������Ϊ________���������д��ڵĻ�ѧ��������________���û��������ʱ����ʧȥ�������________���ж�������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڷ��ֵ�һ����Ȼ��ʮ������������Al��Cu��Fe���ֽ���Ԫ����ɡ��ش��������⣺
(1)����һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ��________�������־��塢����ͷǾ��塣
(2)��̬Feԭ����________��δ�ɶԵ��ӣ�Fe3���ĵ����Ų�ʽΪ______________________________���������軯�ؼ���Fe3�����γɵ���������ɫΪ________��
(3)���Ʊ���Cu(OH)2�ɽ���ȩ(CH3CHO)���������ᣬ��������ԭ��Cu2O����ȩ��̼ԭ�ӵ��ӻ��������Ϊ______________��1 mol��ȩ�����к��еĦҼ�����ĿΪ____________������ķе����Ը�����ȩ������Ҫԭ����__________________________________��Cu2OΪ�뵼����ϣ��������������ڲ���4����ԭ�ӣ�������ԭ��λ�����ĺͶ��㣬��þ�������________��ͭԭ�ӡ�
(4)Al����Ϊ�����������壬�侧������a��0.405 nm����������ԭ�ӵ���λ��Ϊ________����ʽ��ʾAl���ʵ��ܶ�________________________________________________________________________g��cm��3
(���ؼ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���� �� ��
A.40K�� 40Caԭ�������������������������
B.���ʯ��ʯī��������ͬ
C.H2��D2��Ϊͬλ��
D.ij����ֻ��һ��Ԫ�أ�������һ���Ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ���ҽ���������������Ʒ���ձ�������̨����Ȧ�����żܡ�©������Һ©����ʯ�������ƾ��ơ�����������Ͳ��������Բ����ƿ�����ȱ����������Ʒ�ĽǶȿ������ܽ��е�ʵ����Ŀ��(����)
A������ B����ȡ
C��Һ����� D������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com