
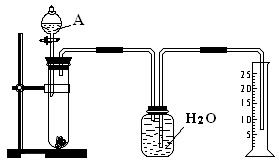
| aL |
| 3”Į22.4L |
| 18a |
| 22.4 |
| ||
| wg |
| 18a |
| 22.4w |
| 18a |
| 22.4w |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś | B£®¢Ū¢Ü | C£®¢Ł¢Ū | D£®¢Ś¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŌŚĶŠÅĢĢģĘ½ÉĻ·ÅĮ½Ę¬“óŠ””¢ÖŹĮæŅ»ŃłµÄÖ½£¬Č»ŗó½«ĒāŃõ»ÆÄĘ·ÅŌŚÖ½Ę¬ÉĻ³ĘĮæ |
| B£®°Ń³ĘµĆµÄĒāŃõ»ÆÄĘ·ÅČėŹ¢ÓŠŹŹĮæÕōĮóĖ®µÄÉÕ±ÖŠ£¬Čܽā”¢ĄäČ“£¬ŌŁ°ŃČÜŅŗŅĘČėČŻĮæĘæ |
| C£®ÓĆÕōĮóĖ®Ļ“µÓÉÕ±”¢²£Į§°ō2-3“Ī£¬Ļ“µÓŅŗŅ²ŅĘČėČŻĮæĘæ |
| D£®ŃŲ²£Į§°ōĶłČŻĮæĘæÖŠ¼ÓČėÕōĮóĖ®£¬µ½æĢ¶ČĻß1-2cmŹ±øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬Ö±µ½ČÜŅŗ°¼ŅŗĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®N2”¢NO”¢NH3 | B£®NH3”¢CO2”¢N2 | C£®NH3”¢CO2”¢NO | D£®NH3”¢N2”¢NO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®æĻ¶ØÓŠSO2”¢CO2ŗĶNO | B£®æĻ¶ØƻӊO2ŗĶNO2 |
| C£®æÉÄÜÓŠCOŗĶCO2 | D£®æĻ¶ØÓŠSO2ŗĶNO |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com