
�ж�����Ԫ��A��B��C��D��E����֪
�� �����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2����
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��
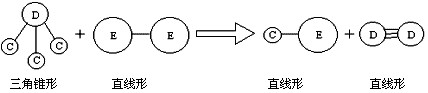
��ش���������
��1�� д��AԪ�صĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4�� BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��д��ѧʽ����
��5��д��Ԫ��E�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
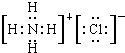
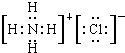
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11�֣��ж�����Ԫ��A��B��C��D��E����֪
�� �����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2����
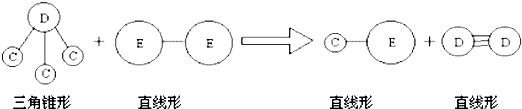
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��
��ش���������
��1�� д��AԪ�صĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4�� BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��д��ѧʽ����
��5��д��Ԫ��E�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10�곤����ʮһ���и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
��11�֣��ж�����Ԫ��A��B��C��D��E����֪
�ٳ����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2����
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��
��ش���������
��1��д��AԪ�صĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4�� BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��д��ѧʽ����
��5��д��Ԫ��E�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ̫ԭ����2010�����5��ĩ�����������ۻ�ѧ ���ͣ�ʵ����
�ж�����Ԫ��A��B��C��D��E����֪��
�ٳ����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ �� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2��
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��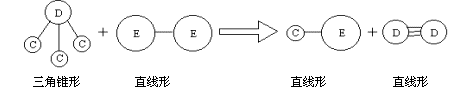
���û�ѧʽ����Ӧ�ķ��Żش��������⣺
��1��д��AԪ�������������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�ǣ� ��
��4��BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��
��5�����û�ѧ����ʽ��ʾEԪ�صĵ����ڹ�ҵ�ϵ�һ����Ҫ��;�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�곤���и߶���ѧ����ĩ���Ի�ѧ�� ���ͣ������
��11�֣��ж�����Ԫ��A��B��C��D��E����֪
�� �����£�AԪ�صĵ����ڿ�����Ũ�����У����涼���������ܵ�����Ĥ��
�� BԪ�ص�ԭ��������AԪ�ش���ԭ�ӵĴ����ĵ�������������������2����
�� E��Aͬ���ڣ�C��D��E����Ԫ���γɵĵ��ʻ���ɷ�������ͼ��ʾ�ķ�Ӧ��
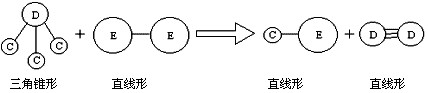
��ش���������
��1�� д��AԪ�صĵ�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��BԪ����Ԫ�����ڱ��� ���ڵ� �壻
B�Ĺ�̬������ľ��������� ��
��3��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��4�� BԪ����EԪ�ص�����������ˮ���������ǿ�� �� ��д��ѧʽ����
��5��д��Ԫ��E�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com