

 __ ����д��ĸ��ţ��м���д��������д��д��0�֣���
__ ����д��ĸ��ţ��м���д��������д��д��0�֣���| A������ | B��BaCl2��Һ | C������ | D��Na2CO3��Һ |
 �˶ۻ�������������________��������Ũ����δ������Ӧ��
�˶ۻ�������������________��������Ũ����δ������Ӧ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 ���������� ��
���������� ��| ʵ�鷽�� | ���ܵ��������Ӧ�Ľ��� |
| ȡ�����ձ��е��ϲ���Һ��װ��A��B��֧�Թ��� | |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
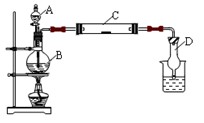

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����



| ѡ�õ�����������ĸ�� | ������Լ� | ���� |
| | | ��Ӧ�����������壩 |
| C | | |
| | ����ͭ | ʹ����������ͭ��Ӧ |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽�� �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����


 I����֤
I����֤ �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��| A��Ba(HCO3)2��Һ | B�������� | C����ˮ | D��Ʒ����Һ |
 �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)�� �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �����ӵ�±ˮΪ��Ҫԭ���Ʊ���ˮ
�����ӵ�±ˮΪ��Ҫԭ���Ʊ���ˮ ��
�� ���������£�
���������£�
 ��
�� ���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ�� ���ữ��ҺZʱ��ʹ�õ��Լ�Ϊ ��
���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ�� ���ữ��ҺZʱ��ʹ�õ��Լ�Ϊ ��
 ���壬����װ���к������� ��
���壬����װ���к������� ��
 �ĵ��볣��
�ĵ��볣�� ��
�� ��
�� �ĵ�
�ĵ� �볣��
�볣�� ��
�� ��ijͬѧ���ʵ����֤
��ijͬѧ���ʵ����֤ ����ǿ��
����ǿ�� ����
����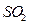 ��
�� ����ֱ�ͨ��ˮ�������ͣ���������ȼƲ�����Һ��
����ֱ�ͨ��ˮ�������ͣ���������ȼƲ�����Һ�� ����ǰ�ߵ�
����ǰ�ߵ� С�ں��ߣ���
С�ں��ߣ��� ����ǿ��
����ǿ�� ����ʵ����Ʋ���ȷ���������� ��
����ʵ����Ʋ���ȷ���������� �� ����ǿ��
����ǿ�� ����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ��� ����������ѡ��
����Ҫ˵��ʵ�鲽�衢����ͽ��ۣ��� ����������ѡ�� ��
��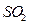 ��
�� ��
�� ��
�� ��
�� ������ˮ������ʯ��ˮ������
������ˮ������ʯ��ˮ������ ��Һ��Ʒ����Һ��
��Һ��Ʒ����Һ�� ��ֽ��
��ֽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��������� | ʵ�鲽�� | ʵ������ | ʵ����� |
| ����2��Сֽ���е����� �ܷ������������� | ȡ����Сֽ���й�������ձ��У���������ˮ���������ڡ� | | ������ ����� |
| ����3���Ҳ�����ʺ�����ʿ�����̼��ƣ��������֤�ҵIJ��룿 | | | �ø������Ʒ����̼��� |
 .
.�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com