
����Ŀ��FeBr2�������л��ϳɵĴ�����ijУͬѧ���ʵ���ø����HBr��Fe��Ӧ�Ʊ�����FeBr2��ʵ��װ������(���ּг�װ����ʡ��)��
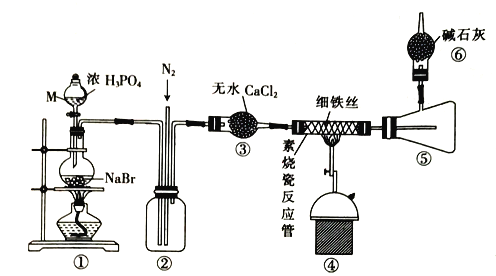
��֪������ʱFeBr3��ֽ�ΪFeBr2��FeBr2����ˮ���⣬800�����Ͽ�������
�ش��������⣺
��1������M��������____________��װ�â�������HBr�Ļ�ѧ����ʽΪ___________��
��2����Ӧ��ʼǰͨ��N2��Ŀ����____________����Ӧ������ͨ��N2��Ŀ����____________��
��3������װ�âܵ������л�������Br2�Բ�Ʒ����_______(��С���û�С�)Ӱ�죬������___________��
��4��װ�â�������___________���ݳ���������Ҫ��___________(�ѧʽ)��
��5�����ʵ�鷽��̽���õ���FeBr2��Fe2+��Br���Ļ�ԭ��ǿ����___________________��
���𰸡�����Һ©�� NaBr��H3PO4![]() NaH2PO4��HBr�� ������������װ���еĿ����ž��� ϡ��HBr�������������װ�� û�� ���ɵ�����FeBr3 �ڸ����»�ֽ�ΪFeBr2 ����ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â� H2��N2 ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ���������۲��������������𰸣�
NaH2PO4��HBr�� ������������װ���еĿ����ž��� ϡ��HBr�������������װ�� û�� ���ɵ�����FeBr3 �ڸ����»�ֽ�ΪFeBr2 ����ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â� H2��N2 ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ���������۲��������������𰸣�
��������
��1������M�������Ƿ�Һ©���������ѻӷ�����H3PO4�ƻӷ�����HBr��������������ƣ�
��2��Ϊ��ֹ����������Ӧ����Ӧ��ʼǰͨ��N2��Ŀ���ǽ�װ���еĿ��������ž�����Ӧ������ͨ��N2��Ŀ����ϡ��HBr�������������װ�á�
��3������Ϣ��֪������ʱFeBr3��ֽ�ΪFeBr2���ʽ���װ�âܵ������л�������Br2�Բ�Ʒ����û��Ӱ�죻
��4���Ƶõ�HBr��Fe��Ӧ����FeBr2��������װ�â���װ�м�ʯ�ң�����ˮ���������壬�����dz�ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â����ݳ���������Ҫ�������͵�����
��5����ԭ��ǿ����Fe2+>Br������������ˮ����˭�ȷ�Ӧ��
��1������M�������Ƿ�Һ©���������ѻӷ�����H3PO4�ƻӷ�����HBr��������������ƣ�װ�â�������HBr�Ļ�ѧ����ʽΪNaBr��H3PO4![]() NaH2PO4��HBr����
NaH2PO4��HBr����
��2����Ӧ��ʼǰͨ��N2��Ŀ���ǽ�װ���еĿ��������ž�����ֹ����������Ӧ����Ӧ������ͨ��N2��Ŀ����ϡ��HBr�������������װ�á�
��3������Ϣ��֪������ʱFeBr3��ֽ�ΪFeBr2���ʽ���װ�âܵ������л�������Br2�Բ�Ʒ����û��Ӱ�죻
��4���Ƶõ�HBr��Fe��Ӧ����FeBr2��������װ�â���װ�м�ʯ�ң�����ˮ���������壬�����dz�ȥβ���е�HBr���������岢��ֹ������ˮ��������װ�â����ݳ���������Ҫ�������͵�������ѧʽΪH2��N2��
��5��Ҫ֤��Fe2+��Br���Ļ�ԭ�Ե�ǿ����ϵ�����Լ�������ˮ����˭�ȷ�Ӧ��ȡ������Ʒ���ھ����������ȴ�������ˮ��ȡ��Һ����������KSCN������CCl4��������ˮ�����������ú�۲��������������𰸣������ϲ���ҺΪѪ��ɫ����Fe2+>Br�������ϲ���Һ��Ϊdz��ɫ���²�Һ��Ϊ�Ⱥ�ɫ����Fe2+��Br����


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʵ������Ҫ����0.1 mol��L��1NaOH��Һ500mL��
��1�����ݼ�����������ƽ��ȡ������Ϊ__________g������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________(�����)����ͼ�����������⣬����������Һ����Ҫ�IJ���������________��
��2������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ������ֻ��һ��)__________��
A��������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��NaOH������ձ��м�������ˮ�ܽ�
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
��3����������������NaOH��ҺŨ��ƫ�ߵ���_____��
A���ܽ����Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶���
II����Ũ����ȡ������Ϊ100 mL��A��B����NaOH��Һ�У��ֱ�ͨ��һ������CO2������������Һ�еμ�0.1 mol/L���ᣬ����CO2�����(��״��)����������������ϵ��ͼ��ʾ��
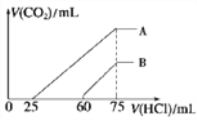
��4����A���߱�����ԭ��Һͨ��CO2���������������ᷴӦ����CO2����������________mL(��״��)��
��B���߱�����ԭ��Һͨ��CO2��������Һ�����ʵĻ�ѧʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Kolbe����ȡ��ϩ��װ����ͼ��ʾ���缫a�ϵIJ���Ϊ��ϩ��̼������ӡ�����˵����ȷ���ǣ� ��

A. ��װ�ý���ѧ��ת��Ϊ����
B. ͼ��Ϊ�����ӽ���Ĥ
C. ������Χ��Һ��pH���ϼ�С
D. ÿ����1mol��ϩ����·��ת��2mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��þ���輰�仯������;�dz��㷺���ش��������⣺
��1����̬Siԭ�Ӽ۲���ӵĵ����Ų�ͼ(�������ʽ)Ϊ__________����̬Mgԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ__________�Ρ�
��2��Mg2C3��ˮ��Ӧ������H2C=C=CH2���м��̼ԭ���ӻ���ʽ��__________����Ӧ���漰��Ԫ���е縺��������__________(��Ԫ�ط���)��Mg2C3��H2C=C=CH2�о�����(����ĸ)__________��
A.��λ�� B.�Ҽ� C.�м� D.���
��3��MgO�����ܿ�ͨ��ͼ1��bom- Haberѭ������õ���
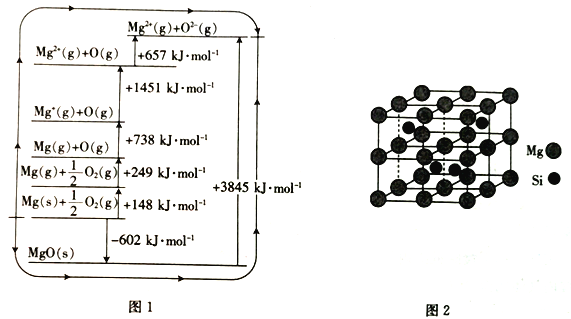
Mg�ĵڶ�������Ϊ__________kJ��mol-1��O=O���ļ���Ϊ_________kJ��mol-1��MgO�ľ�����Ϊ__________kJ��mol-1��
��4��Mg2Si�����ṹ��ͼ2��ʾ����֪���ܶ�Ϊ1.94g��cm-3��NAΪ�����ӵ�������ֵ��
��������a=__________nm(�г�����ʽ)
��Mg2Si����һ�ֱ�ʾ�У��ĸ�Mg������ͼ��ʾ�������е�Si��λ�ã���Si����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����ʵ�鱨�����г������������к������ǣ� ��
A.��10mL��Ͳ��ȡ7.13mLϡ����
B.��pH�Ʋ��ijϡ�����pHΪ1.54
C.�ù㷺pH��ֽ���ij��Һ��pHΪ2.3
D.����100mL1mol/L��NaCl��Һ��������ƽ��ȡ5.85gNaCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ѧ��ӦA2(g)��B2(g)=2AB(g)�������仯��ͼ��ʾ���ж�������������ȷ����()

A. ����1 mol A��A��1 mol B��B�����ų�a kJ����
B. ÿ����2 mol AB(g)����b kJ����
C. �÷�Ӧ�з�Ӧ��������������������������
D. �÷�Ӧ�Ȧ�H��(a��b) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����±��ṩ���ݼ�������ʽṹ֪ʶ����Ӧ1��SiCl4(g)+2H2(g)=Si(g)+4HCl(g)����Ӧ2��Si(g)+O2(g)=SiO2(g)����Ӧ1�ͷ�Ӧ2�ķ�Ӧ��Ϊ
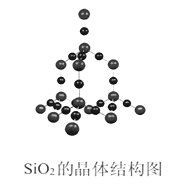 ��ľ���ṹ
��ľ���ṹ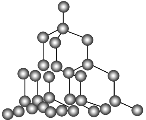
��ѧ�� | Si-Cl | H-H | Si-Si | H-Cl | O=O | Si-O |
����kJ/mol�� | 360 | 436 | 176 | 431 | 498 | 460 |
A. +236kJ/mol��-990kJ/mol B. -116kJ/mol��-990kJ/mol
C. -116kJ/mol��-70kJ/mol D. +236kJ/mol��-70kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������ϢϢ��أ�������������ȷ���ǣ� ��
A.�û���̿��ȥ�������е���ζ
B.���ʹ��ǽ�̫����ת��Ϊ���ܵij��ò���
C.ҽ�þƾ���Ũ��ͨ��Ϊ95%
D.�轺������ʳƷ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�ݺ���FeCl3��FeCl2�Ĺ������Ϊ�ⶨ���ɷֵĺ���������������ʵ�飺
ʵ��1���ٳ�ȡһ����������Ʒ������Ʒ�ܽ⣻
�����ܽ�����Һ�м���������AgNO3��Һ������������
�۽��������ˡ�ϴ�ӡ�����õ���ɫ����17.22 g��
ʵ��2���ٳ�ȡ��ʵ��1����ͬ��������Ʒ������Ʒ�ܽ⣻
�����ܽ�����Һ�У�ͨ��������Cl2��
�������������Һ�м���������NaOH��Һ���õ����ɫ������
�ܽ��������ˡ�ϴ�Ӻ����������������ټ��٣��õ���������4 g��
����ʵ��ش��������⣺
��1���ܽ���������õ��IJ���������________________________��
��2��ʵ��������FeCl2��Һʱͨ���������м��������Լ�________________��
��3����ʵ��2��ͨ������Cl2��Ŀ����_________���漰�Ļ�ѧ��Ӧ�����ӷ���ʽ��__________��
��4�����顰ʵ��2���IJ�����г����Ѿ�ϴ�Ӹɾ��ķ�����________________��
��5������FeCl3��Һ��������Һ����ʱ��ͨ�����ܵõ�FeCl3���壬����ƽ��Ĺ۵������ԭ��ѧ����ʽ������������˵����____________________________________��
��6��FeCl3��Һ��������ֹѪ����Ҫ����ΪFeCl3��Һ��ʹѪҺ�۳������漰�����������ʡ����¹��ڽ����˵����ȷ����________
A������ķ�ɢ��������ֽ
B��ʵ�����Ʊ�����Fe(OH)3���壬�ǽ�����FeCl3��Һ�����ȵ�NaOH��Һ�У���������Һ��Ϊ���ɫ
C��������ͨ������ʱ�ܲ��������ЧӦ
D�����塢��Һ����Һ�У�����ɢ������ֱ�����ķ�ɢϵ�ǽ���
��7��ͨ��ʵ���������ݣ����������Ʒ��FeCl3��FeCl2�����ʵ���֮����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com