
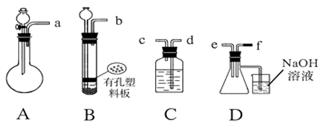
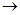 f�����ܿ�������������ȷ��˳����
f�����ܿ�������������ȷ��˳���� | A��befcda | B��adcefb | C��acdfeb | D��acdefb |


 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ά����������ά | B��������Ũ���������������� |
| C���ܽ����ͭ | D��п���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������м���ŨH2SO4����ַ�������˵��ŨH2SO4������ˮ�ԡ� |
| B��ŨH2SO4��ŨHCl��Ͽ��Ƶ��Ȼ��⣬˵��ŨH2SO4��һ���ѻӷ����ᡣ |
| C�������£�Ũ����������������棬˵������ŨH2SO4����Ӧ�� |
| D����ӦCuSO4+H2S �� CuS��+H2SO4�ܽ��У�˵��CuS�Ȳ�����ˮ��Ҳ���������ᡣ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
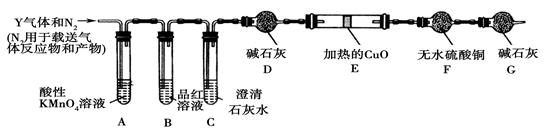
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ũ�����Ũ���ᶼ��������ֱ�Ӹ�п����Ӧ������ |
| B��Ũ�����Ũ���ᶼ�������ͭ��Ӧ |
| C��Ũ�����Ũ�����ˮϡ�ͺ��������ͭ��Ӧ |
| D��Ũ�����Ũ�����ڳ����¶����ý���������������ʢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SO2+H2O=H2SO3 | B��SO2+2NaOH=Na2SO3+H2O |
| C��2SO2+O2=2SO3 | D��SO2+CaO=CaCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��ͨ��Ʒ����Һ | B��ͨ������ʯ��ˮ |
| C����ͨ�����������Һ,��ͨ������ʯ��ˮ | D����ͨ������ʯ��ˮ, ��ͨ�����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
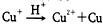 ����NH4CuSO4�м�������3mol/L���������������ȷ����
����NH4CuSO4�м�������3mol/L���������������ȷ����| A���д̼�����ζ�İ������� |
| B������1mol NH4CuSO4�μӷ�Ӧ����ת��3mol e�� |
| C�������ڷ�Ӧ���������� |
| D��������Һ����ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com